Cumulative fluid balance and mortality in elderly patients with acute kidney injury requiring continuous renal-replacement therapy: a multicenter prospective cohort study
Article information
Abstract
Background
The effect of fluid balance on outcomes in elderly patients with acute kidney injury (AKI) requiring continuous renal-replacement therapy (CRRT) is not explained well. We investigated outcomes according to cumulative fluid balance (CFB) in elderly patients with AKI undergoing CRRT.
Methods
A total of 607 patients aged 65 years or older who started CRRT due to AKI were enrolled and stratified into two groups (fluid overload [FO] vs. no fluid overload [NFO]) based on the median CFB value for 72 hours before CRRT initiation. Propensity score-matching analysis was performed.
Results
The median age of included patients was 73.0 years and 60.0% of the population was male. The median 72-hour CFB value was 2,839.0 mL. The overall cumulative survival and 28-day survival rates were lower in the FO group than in the NFO group (P < 0.001 for both) and remained so after propensity score-matching. Furthermore, patients in the FO group demonstrated a higher overall mortality risk after adjustment for age, sex, systolic blood pressure, Charlson comorbidity index, Acute Physiology and Chronic Health Evaluation II score, serum albumin, creatinine, diuretic use, and mechanical ventilation status (hazard ratio, 1.38; 95% confidence interval, 1.13 to 1.89; P < 0.001). Among survivors, both the duration of CRRT and the total duration of hospitalization from CRRT initiation showed no difference between the FO and NFO groups.
Conclusion
A higher CFB value is associated with an increased risk of mortality in elderly patients with AKI requiring CRRT.
Introduction
Continuous renal-replacement therapy (CRRT) has become an essential strategy for the management of critically ill patients with acute kidney injury (AKI) [1,2]. Previous research has identified several prognostic factors for mortality—including sepsis, higher Acute Physiology and Chronic Health Evaluation (APACHE) II score, oliguria, higher Sequential Organ Failure Assessment (SOFA) score, and the need for mechanical ventilation—in patients with severe AKI undergoing CRRT [3-6]. However, predicting the outcome of patients requiring CRRT remains a challenge. Recent studies have focused on the impact of cumulative fluid balance (CFB) on the outcomes of patients with severe AKI and have suggested that such could also be an important factor affecting outcomes in this patient group [7-12].
Elderly patients are prone to AKI onset owing to their renal structural and functional alterations, multiple accompanying comorbidities, and polypharmacy [13-17]. Recent evidence suggests that the effect of fluid status on outcome might be different between elderly patients and the general population. Because of their decreased autonomic function and reduced cardiovascular reserve, elderly patients undergoing CRRT are commonly exposed to the risk of overhydration. However, at the same time, the aforementioned factors may also cause elderly patients to be more susceptible to hydration-related complications such as intradialytic hypotension and arrhythmias. Moreover, changes in hydration status could result in alterations in serum electrolytes and osmolality, further compromising the outcomes [18,19]. Nevertheless, few studies to date have specifically examined whether the CFB has a certain effect on elderly patients with AKI requiring CRRT.
In this study, through an analysis of a multicenter, prospective CRRT cohort, the impact of CFB on mortality risk was evaluated in elderly patients with AKI requiring CRRT.
Methods
Study subjects
From a multicenter cohort (Seoul National University Hospital, Seoul National University Boramae Medical Center, and Severance Hospital), we initially screened a total of 1,471 adults (defined as those aged ≥ 18 years) in whom CRRT was initiated in the intensive care unit (ICU) because of AKI between August 2009 and December 2013. Those who were aged younger than 65 years and who had undergone chronic maintenance dialysis were excluded; a total of 607 patients were finally analyzed. AKI was defined based on the Kidney Disease: Improving Global Outcomes clinical practice guidelines for AKI, as follows: an increase in serum creatinine by 0.3 mg/dL or more (≥ 26.5 µmol/L) within 48 hours, an increase in serum creatinine to at least 1.5 times the baseline value known or presumed to have occurred within the previous seven days, or a urine volume of less than 0.5 mL/kg/hr for six hours. For the diagnosis of systemic inflammatory response syndrome, at least two of the following criteria were required: core temperature of either 38°C or greater or 36°C or less, heart rate of more than 90 beats/min, respiratory rate of higher than 20 breaths/min, PCO2 of 32 mmHg or less or mechanical ventilator use, and peripheral leukocyte count of either 12,000/mm3 or greater or 4,000/mm or less [20]. Septic AKI was defined as a systemic inflammatory response syndrome combined with an infectious episode and AKI. The study subjects were divided into the fluid overload (FO) and no fluid overload (NFO) groups based on the median value of CFB during 72 hours before CRRT initiation. All included patients were followed up with until the time of death or hospital discharge. The study protocol complied with the Declaration of Helsinki guidelines and received full approval from the Institutional Review Boards at Seoul National University Hospital, Seoul National University Boramae Hospital, and Severance Hospital. We obtained informed consent from the participants prior to their enrollment in the present study.
Data collection
The demographic and baseline clinical characteristics of the enrolled patients were collected at the time of CRRT initiation, including age, sex, body mass index, cause of AKI, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), Charlson comorbidity index (CCI), and SOFA and APACHE II scores. In addition, the requirements for mechanical ventilation and the fraction of inspired oxygen were investigated, while the prescriptions for CRRT including target clearance, dialysate flow rate, replacement flow rate, and use of anticoagulation were also assessed. Laboratory data were collected at the initiation of CRRT and the estimated glomerular filtration (eGFR) was calculated using the Modification of Diet in Renal Disease equation [21].
The causes of AKI (i.e., septic, nephrotoxic, ischemic, postoperative, or other) were initially categorized based on a review of the electronic medical records performed by the researchers and confirmed by clinical diagnosis [22]. Nephrotoxic AKI was diagnosed as drug-induced AKI if offending drugs were administered within at least two weeks prior to the onset of AKI and if all other contributing factors to AKI were absent. If more than one contributing factor was found to be occurring simultaneously, the major factors that had contributed to AKI were determined via clinical judgment rendered by researchers who were blinded to the clinical outcomes.
The initiation of CRRT and the prescription of CRRT settings were determined through consultations with nephrologists [23]. The criteria for CRRT initiation were medically intractable or persistent electrolyte imbalance, metabolic acidosis, decreased urine output with volume overload and/or progressive azotemia, and hemodynamic instability in patients with a diagnosis of AKI. Generally, the vascular access for CRRT was established using a femoral venous catheter and the predilution method of continuous venovenous hemodiafiltration was adopted. To maintain CRRT adequacy after initiation, the attending physicians and experienced nurses monitored each patient’s body weight, urine output, laboratory results, actual delivered dose, and hemodynamic status and discussed the results with nephrologists.
Fluid status assessment and clinical outcomes
All available intake and output data for the 72 hours immediately before CRRT initiation and the sum of this data were used to calculate the CFB. The input data included the amount of intravenous infusion of normal saline, dextrose, total parenteral nutrition, transfused material, or oral feedings. The output data included urine and other body fluids excreted from the catheter. The primary outcome was all-cause mortality after CRRT initiation. The secondary outcomes were 28-day mortality after CRRT initiation, durations of CRRT and total renal-replacement therapy (RRT), and durations of ICU stay and total hospitalization from the initiation of CRRT.
Statistical analysis
The baseline characteristics of the groups were compared using the independent t test for continuous variables and the chi-squared test for categorical variables, respectively. Continuous variables are presented as mean ± standard deviation and categorical variables are presented as numbers and percentages. After a test for normality, non-normally distributed variables were expressed as median (interquartile range) and were compared using the Mann-Whitney U or Kruskal-Wallis test. Patient survival was estimated using Kaplan-Meier curves and multivariate Cox regression models based on the CFB findings during 72 hours before CRRT initiation. Propensity scores were estimated using multiple logistic regression analysis with adjustments for age, sex, CCI, SBP, and serum creatinine level. After calculating the propensity scores, we matched patients in the FO and NFO groups with similar propensity scores at a 1:1 ratio, using the nearest-neighbor method without replacements and with a 0.2-caliper width. Propensity score-matching (PSM) was used to increase the precision of the estimated effect without increasing the bias resulting from the presence of variables potentially associated with survival [24]. The characteristics of both the FO and NFO groups were compared before and after PSM. Kaplan-Meier survival curves and life tables were generated for the two groups after PSM.
All statistical tests were conducted using a two-tailed 95% confidence interval (CI) and a value of P < 0.05 was considered to be statistically significant. All descriptive and survival analyses were performed using the IBM SPSS ver. 25.0 for Windows software program (IBM Corp., Armonk, NY, USA). Meanwhile, the R ver. 3.1.0 software program (R Foundation for Statistical Computing, Vienna, Austria) was used for PSM.
Sensitivity analyses
We adjusted the CFB by body weight at ICU admission to quantify the fluid overload percentage (FO%), which was defined as the percentage of fluid accumulation by dividing CFB in liters by patient’s body weight at ICU admission and multiplying by 100%. We divided subjects into high and low FO% groups based on the median value of FO% and adopted the groups as independent variables in multivariable Cox regression modeling for all-cause mortality.
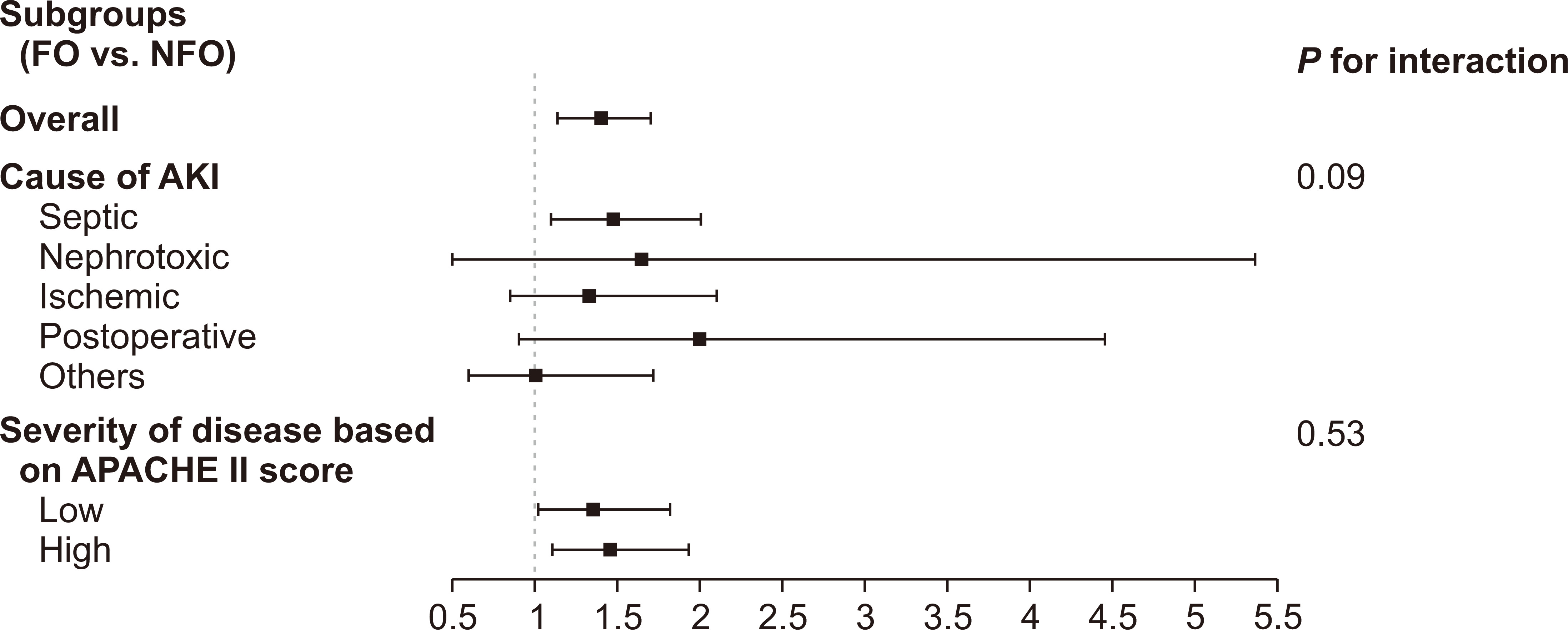
We further performed subgroup analysis to stratify the mortality risk according to the causes of AKI (i.e., septic, nephrotoxic, ischemic, postoperative, and other) and the severity of disease based on the median value of APACHE II score (high vs. low).
Results
Baseline characteristics
The baseline characteristics of the study patients at CRRT initiation are presented in Table 1. The median age was 73.0 years and 60.0% of the patients were male. Sepsis (45.3%) was the most common cause of AKI, followed by ischemia (18.5%), postoperative complications (9.7%), and nephrotoxicity (7.4%), respectively. The median CFB value was 2,839.0 mL. As noted, the patients were divided into the FO (n = 303) vs. NFO (n = 304) groups according to the median CFB value of the total group (2,839.0 mL [1,892.1-4,631.8 mL]). Meanwhile, the median CFB value was 5,344.0 mL (3,784.0-7,274.0 mL) in the FO group and 1,028.5 mL (0.0-1,989.7 mL) in the NFO group. No differences were observed between the two groups with respect to the six-hour urine volume before CRRT initiation or in dialysis parameters including target clearance, dialysate flow rate, replacement flow rate, and blood flow rate. The time from ICU admission to CRRT initiation was 12.6 hours (1.9-50.7 hours) in the FO group and 3.9 hours (1.4-20.5 hours) in the NFO group (P < 0.001). The CCI was higher in the NFO group, whereas the SOFA score, APACHE II score, and proportion of patients requiring mechanical ventilation were higher in the FO group (P < 0.001). Further, the SBP, DBP, and MAP were lower in the FO group and diuretic use was more frequent in the FO group. In the laboratory tests, the FO group showed lower serum creatinine and albumin levels, hemoglobin values, and platelet counts but higher eGFR, aspartate aminotransferase levels, and alanine aminotransferase levels. The white blood cell count, prothrombin time–international normalized ratio (PT-INR), and serum total bilirubin and blood urea nitrogen (BUN) levels were comparable between the two groups.
The patients in the two groups were matched using propensity scores for CFB levels, encompassing the following covariates: age, sex, CCI, SBP, and serum creatinine level. After PSM, a total of 520 patients (260 in each group) remained. The propensity scores of the matched patients were not different between the two groups. Most baseline parameters, including age, sex, body mass index, CCI, SOFA score, SBP, DBP, MAP, six-hour urine volume before CRRT initiation, dialysis prescriptions, and biochemical data except for the PT-INR and serum total bilirubin level were comparable between the FO and NFO groups. However, in the FO group, time from ICU admission to CRRT initiation was shorter and the APACHE II score and rate of mechanical ventilation requirement or diuretic usage were higher than in the NFO group. Meanwhile, the PT-INR, total bilirubin level, and creatinine level were lower in the FO group than in the NFO group.
Association between CFB and all-cause mortality
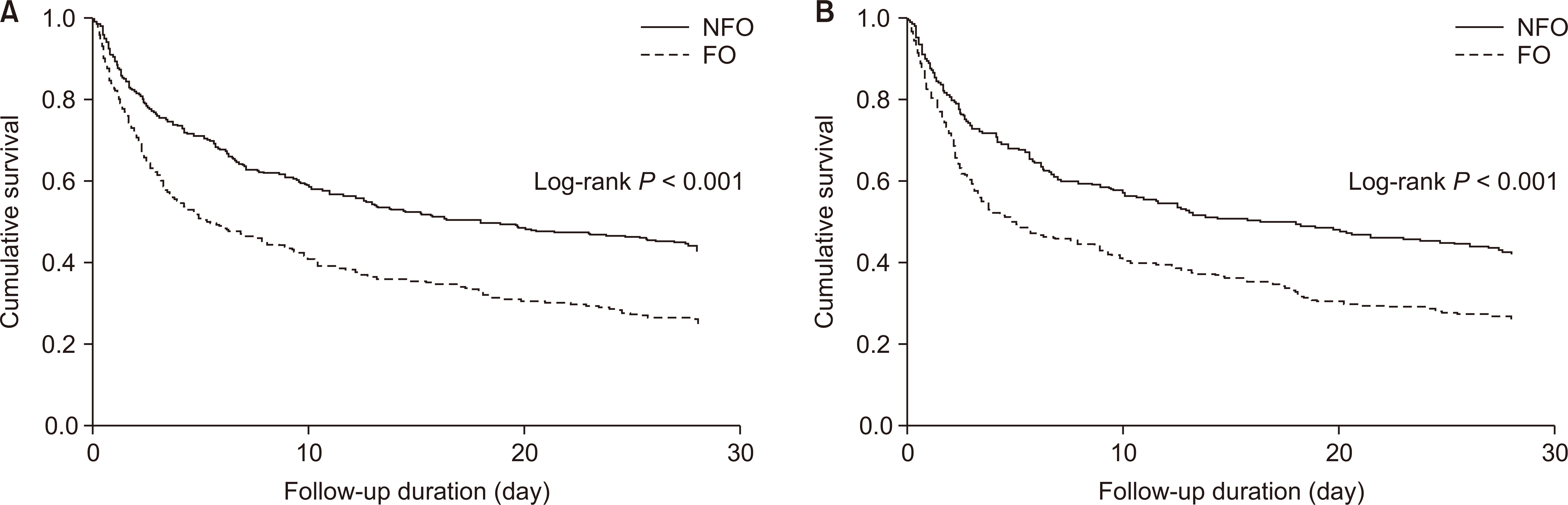
A total of 490 (79.2%) deaths occurred during the median follow-up period of 9.6 days. Fig. 1 shows the Kaplan–Meier curves for survival rates between the FO and NFO groups before (A) and after (B) PSM. The risk of all-cause mortality was significantly lower in the FO group than in the NFO group. This result remained consistent after PSM (P < 0.001). Multivariable Cox proportional-hazards analysis was also performed with or without PSM (Table 2). Before PSM, the FO group showed an increased risk of mortality in the univariable model (model 1) (hazard ratio [HR], 1.74; 95% CI, 1.45-2.08; P < 0.001). This association was significant after adjustment for age, sex, and CCI (model 2) (HR, 1.71; 95% CI, 1.42-2.05; P < 0.001) or for SBP, serum albumin and creatinine levels, APACHE II score, use of diuretics, mechanical ventilation status (model 3) (HR, 1.38; 95% CI, 1.13-1.89; P < 0.001). Moreover, this association remained significant after PSM (model 4) (HR, 1.59; 95% CI, 1.31-1.94; P < 0.001). The 28-day cumulative survival was lower in the FO group (P < 0.001; Fig. 2A), and this trend also remained significant after PSM (P < 0.001; Fig. 2B).

Kaplan-Meier curves for all-cause mortality compared between the fluid overload (FO) and no fluid overload (NFO) groups before (A) and after (B) propensity score-matching.
Duration of RRT and hospitalization
Before PSM, the duration of CRRT was 3.1 days (1.3-6.2 days) in the FO group and 3.4 days (1.4-6.9 days) in the NFO group (P = 0.287; Table 3). The duration of total RRT and the total duration of hospitalization were shorter in the FO group (3.4 and 7.2 days, respectively) than in the NFO group (4.7 and 17.8 days, respectively) (P = 0.032 and P < 0.001, respectively). However, among the survivors, no differences were observed in the duration of CRRT, total RRT, ICU stay, or hospitalization between the FO and NFO groups.
After PSM, no significant difference was found in the duration of CRRT or total RRT between the total study group and the survivors. The duration of ICU stay from CRRT initiation was also not different between the two groups. However, although the total duration of hospitalization was shorter in the FO group, no difference was observed among the survivors of the two groups.
Sensitivity analyses
We further conducted an analysis using the adjusted CFB by body weight at ICU admission (FO%). Both a multivariable Cox analysis for all-cause mortality and a PSM analysis were performed and the results were consistent with the main findings that subjects with high FO% experienced an increased risk of mortality as compared with those with low FO% (Table 4).
In addition, we stratified the risk according to causes of AKI (septic, nephrotoxic, ischemic, postoperative, and other) and the severity of disease according to the APACHE II score (high vs. low based on the median value). The subgroups and fluid status showed no interaction with mortality, suggesting that the relationship between CFB and outcome remained regardless of AKI cause and patient severity (Fig. 3).
Discussion
In this study, we compared the survival rates between the FO and NFO groups based on CFB assessed at 72 hours before CRRT initiation in elderly patients undergoing CRRT because of AKI. In the FO group, the risks of all-cause mortality and 28-day mortality were significantly higher than those in the NFO group. These results were consistent after the PSM analysis. Among survivors, the duration of CRRT or hospitalization showed no difference between the FO and NFO groups. These results indicate that the risk of mortality increases according to the amount of fluid used for resuscitation prior to CRRT initiation.
Previous studies have identified prognostic factors for mortality in patients with severe AKI requiring RRT. In the early 2000s, several study groups found that continuous and intensive RRT was related to a lower mortality risk than intermittent hemodialysis in patients with AKI [25,26]. Several years later, an observational cohort study investigated the prognostic factors of critically ill patients treated with CRRT in the ICU [4]. A more recent investigation of 197 patients requiring CRRT initiation in the ICU setting identified three independent factors associated with fatal outcomes (mechanical ventilation requirement, sepsis, and septic shock requiring vasoactive agents) and two factors associated with favorable outcomes (nonoliguric AKI and low serum creatinine levels). Finally, several study groups have attempted to identify more reliable prognostic factors associated with worse outcomes in patients with AKI, including disease severity assessed according to the APACHE II or SOFA score, amount of urine output, serum BUN level, timing of CRRT, and previous health status [3-6,27-29]. Despite improvements in the knowledge of such prognostic factors in patients requiring CRRT, the mortality rate of patients with AKI treated with CRRT remains high, reaching nearly 50% [30,31]. Furthermore, controversies remain about which factor could best predict clinical outcomes. In this regard, it is difficult to conclude that the prognostic factors in patients with AKI requiring CRRT have been completely identified. Therefore, there is considerable interest in developing novel management strategies for reducing the mortality rate.
Recently, several studies have reported that FO during the ICU stay could be an important prognostic factor for predicting mortality in patients with AKI treated with CRRT [7-12]. Fluid administration is inevitable in ICU patients with septic shock or AKI; however, fluid resuscitation may enhance filling pressure and improve microcirculation in these conditions [9,32]. Nevertheless, several studies focused on the undesirable effects of fluid administration and demonstrated that a positive mean daily fluid balance was associated with increased mortality in patients with AKI treated in the ICU. One study group found that a positive fluid balance was associated with a higher risk of mortality in ICU patients with AKI [11], while another study similarly confirmed the association of higher fluid balance with mortality in ICU patients with AKI [33]. These studies reported a higher mean fluid balance existed in nonsurvivors than in survivors during the first seven days of ICU stay. However, both studies had several limitations. First, both study groups included patients with AKI regardless of CRRT initiation. Because the fluid status might be different between patients with and without CRRT, respectively, the analysis of these populations should be performed separately. Also, the latter study included only 132 participants with AKI and the definition of AKI used for inclusion in the analysis was based on a serum creatinine level of 3.5 mg/dL or higher or a urine output of less than 500 mL/day, without the use of a baseline serum creatinine level to assess the absolute or relative changes in serum creatinine. Moreover, both studies defined fluid status as the mean value of daily fluid balance and, importantly, the use of this value could cause miscalculation or underestimation of the exact fluid status. It is crucial to define FO by using a decisive rather than arbitrary method.
CFB is defined as the net fluid that accumulates during the first 72 hours of CRRT or the ICU stay [7]. This definition is widely accepted and provides clinically valuable information to help make risk stratification more uniform among critically ill patients, with CFB incorporated as an additional clinical parameter [12]. An observational study that investigated 618 ICU patients with AKI evaluated the association between FO and mortality as well as the recovery of kidney function [10]. The authors defined FO as an increase of more than 10% in CFB adjusted for body weight relative to baseline and reported that FO was significantly related to a higher risk of mortality, not to kidney function recovery. Notably, these authors studied large numbers of patients and obtained well-defined measurements of fluid status; however, the study groups were heterogeneous, as half of the patients were treated with CRRT and half were treated without CRRT. Later studies found that a higher CFB at the time of dialysis initiation for AKI was associated with mortality [34] and worse renal recovery [35]. One prospective, multicenter, observational study evaluated the association between FO at RRT initiation and mortality in 283 critically ill patients with AKI and reported that CFB was associated with an increased risk of 90-day mortality [34]. Another study showed that FO could predict worse renal recovery in hospitalized patients with AKI requiring RRT [35]. In this single-center study with 170 subjects, CFB was a significant negative predictor of renal recovery. These studies evaluated the negative effect of FO on worse outcomes in critically ill patients with AKI, yet the population sizes of these studies were relatively small. Moreover, the age group, which is one of the most important prognostic factors for clinical outcomes in patients with AKI, was heterogeneous.
The elderly population, which includes individuals aged 65 years or older, is rapidly growing in developed countries alongside an increase in the global life expectancy [36]. In accordance with the increasing size of the elderly population, the incidence of AKI is also rising [15,37,38]. Elderly people have a higher tendency to develop AKI because of structural or functional alterations in the kidneys, the presence of multiple comorbidities (e.g., arteriosclerosis, hypertension, diabetes mellitus, and heart failure), and resultant medication use [13,14,39]. Thus, the elderly population is less able to adapt to rapid hemodynamic changes and alterations in electrolyte levels and osmolarity [16,17,40]. Furthermore, maintaining a stable fluid balance is difficult for elderly patients. Finally, the importance of fluid balance might be as essential for elderly individuals as it is for the general population. To our knowledge, this study is the first to investigate the association between CFB and mortality among elderly patients with AKI treated with CRRT. This study has strength in that it included 607 elderly patients with AKI requiring CRRT and evaluated large numbers of patients with equivalent treatment conditions. Further, a PSM analysis with more than 400 patients was additionally performed.
However, this study also has several limitations. First, because of its observational design, the causality between FO and worse clinical outcomes cannot be clearly determined. Fluid itself, as is true with any medication used in ICU care, may have harmful effects, which could indicate that the association between FO and mortality is largely secondary. Randomized controlled studies should be performed to overcome this limitation. Second, there was no benefit of lower CFB on the duration of RRT or hospitalization, which was more evident as a result after PSM analysis. We assumed that because patients with FO obviously showed shorter survival days, they also demonstrated shorter durations of RRT and hospitalization relative to patients with NFO. Third, although we adjusted for various confounding factors using multivariable analyses, residual confounding by unmeasured covariates may not have been completely eliminated. However, we performed a PSM analysis to overcome this limitation. Finally, our results do not allow us to define the appropriate amount of fluid. Despite the adverse effects of FO on clinical outcomes, determining the optimal amount of fluid administration remains a challenge in ICU care, especially in elderly patients with AKI. Thus, further studies are warranted to identify a clear target amount of fluid administration so as to minimize the negative effects and maximize the benefits of fluid management.
In conclusion, an increasing CFB is associated with an increased risk of mortality in elderly patients with AKI requiring CRRT. Our study results emphasize that close observation of the fluid balance is important and that use a large amount of fluid for resuscitation before CRRT can result in poor survival in elderly patients with AKI.
Acknowledgments
The data used in this study were obtained from Seoul National University Hospital, Seoul National University Boramae Medical Center, and Severance Hospital in Seoul, Republic of Korea.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant no. HC15C1129).
Authors’ contributions
Jong Hyun Jhee, Jae Yoon Park, Dong Ki Kim, Seung Hyeok Han, Tae-Hyun Yoo, Shin-Wook Kang, and Jung Tak Park participated in the data collection. Jong Hyun Jhee, Jae Yoon Park and wrote the manuscript. Jung Pyo Lee and Jung Tak Park participated in the study design. Jong Hyun Jhee, Jae Yoon Park, Jung Nam An performed the statistical analysis. Jong Hyun Jhee, Jae Yoon Park, Jung Pyo Lee, and Jung Tak Park participated in the conception, analysis, and interpretation of data. Kwon Wook Joo, Yun Kyu Oh, Chun Soo Lim, Yon Su Kim, and Shin-Wook Kang participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.