| Kidney Res Clin Pract > Volume 31(1); 2012 > Article |
|
Functional iron deficiency (FID), referring to an impaired provision of iron needed for erythropoiesis despite adequate iron storage in the body (serum ferritin>100 μg/L and transferrin saturation (TSAT)>20%), is commonly observed in dialysis patients, being found in about 20% of those patients according to some European reports [1], [2]. FID in dialysis patients was vaguely known to be caused by chronic inflammatory status and kidney dysfunction until the discovery of hepcidin explained a large part of the pathophysiological mechanism [3].
Dialysis patients showed a significantly skewed distribution and a wide variation of plasma vitamin C levels. In one study, 15% of the hemodialysis (HD) patients had a severe vitamin C deficiency (<10 μM). A higher plasma vitamin C is associated with a lower plasma parathyroid hormone (PTH) [4]. The effectiveness of vitamin C as an adjuvant therapy for anemia in patients receiving erythrocyte-stimulating agent (ESA) or intravenous iron has long been known. It was already shown in 1995 that in dialysis patients receiving transfusion, intravenous vitamin C improved the anemia status which worsened again after discontinuation of vitamin C [5]. Later studies have shown that vitamin C treatment increased blood hemoglobin level, thereby reducing the ESA dose requirement [6], [7]. Hypochromic red blood cells (HRC) reflect iron adequacy for erythropoiesis. A higher HRC percentage is known to be associated with a better response to vitamin C [8]. The direct mechanism, however, behind the effect of vitamin C on anemia is not clear yet. One hypothesis is that vitamin C affects mobilization of stored iron from the Kupffer cells in the liver and other sites in the reticuloendothelial system, increasing release of iron available for erythropoiesis [9].
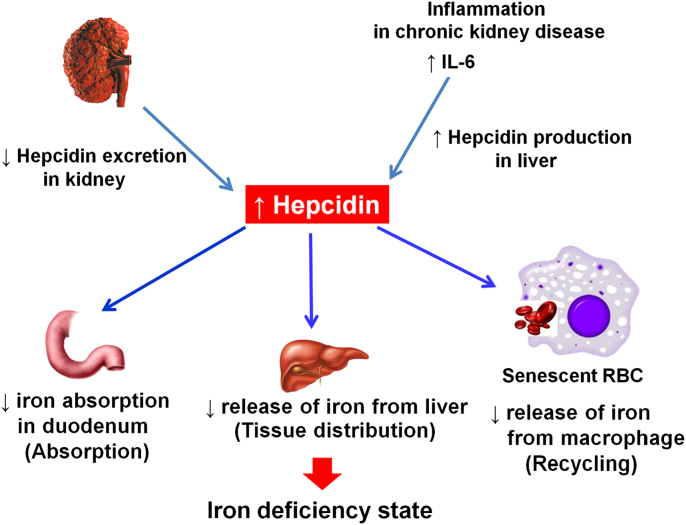
Since its first description in 2000, hepcidin, a peptide hormone synthesized in the liver, has been shown to be the central regulatory module for systemic iron homeostasis [10]. Hepcidin is in part removed from the body by glomerular filtration and degradation in the proximal tubule [11]. With diminishing renal function, the renal excretion and degradation of hepcidin is reduced while its hepatic production is increased by the inflammatory stimuli marked by elevated interleukin 6 (IL-6) [3]. As a result, the blood hepcidin level is increased in dialysis patients. Hepcidin in the human body controls absorption, recycling, and tissue distribution of iron by decreasing (1) iron absorption from the duodenum; (2) iron release from the macrophages involved in recycling senescent red blood cells; and (3) iron release from iron-storing hepatocytes. The net result of elevated hepcidin is decreased iron transfer into blood plasma, leading to FID [10] (Fig. 1). The hepatic production of hepcidin is increased by interleukin-6 (IL-6). Vitamin C counters inflammation as a strong anti-oxidant and thus is believed to be involved in hepcidin production, too. No study is available, however, to support a direct association between vitamin C and hepcidin, except that alcohol represses the expression of hepcidin messenger RNA, and that vitamin C increases hepcidin expression in the liver of patients with alcoholic liver disease [12]. Further research is needed to examine the association between vitamin C treatment and hepcidin in FID anemia of dialysis patients.
In dialysis patients, the two main axes of anemia treatment are ESA and intravenous iron therapy, each with respective problems. ESA is costly and associated with increased risk of tumor growth, pure red cell aplasia, cardiovascular complications including myocardial infarction and stroke, thrombosis and hypertension. Ultimately, excess treatment with ESA might raise mortality [13], [14], [15]. On the other hand, intravenous iron therapy poses the risk of anaphylaxis, oxidative stress, and infection [16].
In this issue of Kidney Research and Clinical Practice, Kang et al [17] report that intravenous vitamin C is effective on erythropoietin-resistant normoferritinemic anemia in hemodialysis patients. Intravenous (IV) administration of vitamin C (500 mg IV in each dialysis session for 3 months) in the iron-replete normoferritinemic hemodialysis patients (serum ferritin, 100–500 μg/L) was associated with an increased hemoglobin level and a lower weekly erythropoietin requirement in 61% of the patients. In the vitamin C-responsive group of patients, serum iron levels and TSAT scores went up whereas the serum ferritin decreased, indicative of mobilization of stored iron. With such results on the treatment efficacy of vitamin C, one might suggest that vitamin C lowers the risk of exposure to ESA or iron and also the cost of treatment when used as an adjuvant therapy for anemia. Attention, however, must be drawn to some of the details of this particular study: (1) the sample size is a little too small (control group, n=25; treated group, n=33) and the accuracy of the study design is less than desirable, which makes bias evaluation difficult; (2) lack of data on the amount of iron supplements used during the study; (3) lack of data on intact PTH during the study period, since hyperparathyroidism is one of the culprits for ESA resistance, and there is a report that vitamin C is associated with decreased PTH [4]; and (4) unclear description of the target hemoglobin level and lack of evidence on which treatment response was defined. In addition, there was no measurement provided of blood vitamin C or hepcidin levels.
In dialysis patients, dietary intake of vitamin C gets easily insufficient. Foods rich in vitamin C, such as fruit juice and broccoli, also contain plenty of potassium, a nutrient restricted for these patients. As a result, vitamin C deficiency can readily occur [18], and matters get worse given the heavy losses of vitamin C during hemodialysis [19]. Use of vitamin C supplements also requires caution. Vitamin C is metabolized into oxalic acid, and excess oxalate accumulation leads to formation of oxalate crystal deposits in the tissues [9]. Indeed, clinical trials using vitamin C have generally been designed to be short term (90–180 days) to avoid oxalosis [20]. It is necessary to have a further large-scale, prospective, randomized clinical trial on the long-term safety and efficacy of vitamin C as an adjuvant therapy for anemia.
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
References
1. Valderrábano F., Hörl W.H., Macdougall I.C., Rossert J., Rutkowski B., Wauters J.P.. PRE-dialysis survey on anaemia management. Nephrol Dial Transplant. 18:2003;89–100.


3. Hentze M.W., Muckenthaler M.U., Galy B., Camaschella C.. Two to tango: regulation of mammalian iron metabolism. Cell 142:2010;24–38.


4. Richter A., Kuhlmann M.K., Seibert E., Kotanko P., Levin N.W., Handelman G.J.. Vitamin C deficiency and secondary hyperparathyroidism in chronic haemodialysis patients. Nephrol Dial Transplant. 23:2008;2058–2063.


5. Gastaldello K., Vereerstraeten A., Nzame-Nze T., Vanherweghem J.L., Tielemans C.. Resistance to erythropoietin in iron-overloaded haemodialysis patients can be overcome by ascorbic acid administration. Nephrol Dial Transplant. 10:1995;44–47.

6. Tarng D.C., Huang T.P.. A parallel, comparative study of intravenous iron versus intravenous ascorbic acid for erythropoietin-hyporesponsive anaemia in haemodialysis patients with iron overload. Nephrol Dial Transplant. 13:1998;2867–2872.


7. Attallah N., Osman-Malik Y., Frinak S., Besarab A.. Effect of intravenous ascorbic acid in hemodialysis patients with EPO-hyporesponsive anemia and hyperferritinemia. Am J Kidney Dis. 47:2006;644–654.


8. Sezer S., Ozdemir F.N., Yakupoglu U., Arat Z., Turan M., Haberal M.. Intravenous ascorbic acid administration for erythropoietin-hyporesponsive anemia in iron loaded hemodialysis patients. Artif Organs 26:2002;366–370.


9. Handelman G.J.. Vitamin C neglect in hemodialysis: sailing between Scylla and Charybdis. Blood Purif. 25:2007;58–61.


11. Zaritsky J., Young B., Wang H.J., Westerman M., Olbina G., Nemeth E., Ganz T, Rivera S., Nissenson A.R., Salusky I.B.. Hepcidin—a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 4:2009;1051–1056.



12. Guo X., Li W., Xin Q., Ding H., Zhang C., Chang Y., Duan X.. Vitamin C protective role for alcoholic liver disease in mice through regulating iron metabolism. Toxicol Ind Health 27:2011;341–348.


13. Krapf R., Hulter H.N.. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin J Am Soc Nephrol. 4:2009;470–480.


14. Fishbane S., Besarab A.. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2:2007;1274–1282.


15. Bennett C.L., Silver S.M., Djulbegovic B., Samaras A.T., Blau C.A., Gleason K.J., Barnato S.E., Elverman K.M., Courtney D.M., McKoy J.M., Edwards B.J., Tigue C.C., Raisch D.W., Yarnold P.R., Dorr D.A., Kuzel T.M., Tallman M.S., Trifilio S.M., West D.P., Lai S.Y., Henke M.. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. J Am Med Assoc. 299:2008;914–924.

16. Hörl W.H.. Clinical aspects of iron use in the anemia of kidney disease. J Am Soc Nephrol. 18:2007;382–393.


17. Kidney Res Clin Pract.
19. Morena M., Cristol J.P., Bosc J.Y., Tetta C., Forret G., Leger C.L., Delcourt C., Papoz L., Descomps B., Canaud B.. Convective and diffusive losses of vitamin C during haemodiafiltration session: a contributive factor to oxidative stress in haemodialysis patients. Nephrol Dial Transplant. 17:2002;422–427.


- TOOLS
-
METRICS

- Related articles
-
Predicting the probability of survival in acute paraquat poisoning
☆ 2016 June;35(2)Renal replacement therapy in Korea, 2012
☆ 2014 March;33(1)Clostridium difficile- associated diarrhea in dialysis patients☆ 2013 March;32(1)Brief Report: Renal replacement therapy in Korea, 2010
☆ 2012 March;31(1)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















