Introduction
Renal artery stenosis (RAS) is frequently associated with hypertension and renal insufficiency [1]. Nephrotic-range proteinuria is usually caused by primary or secondary glomerular diseases associated with diabetes, drugs, malignancy, infectious disease, or autoimmune disease [2]. Although proteinuria caused by RAS has been reported in some cases, it is uncommon to cause significant heavy proteinuria [3], [4]. During recent decades, ongoing research has pursued treatment options for renovascular disease, focusing on the effectiveness of medical therapy and endovascular intervention [5], [6], [7], [8], [9]. Previous clinical studies showed that renal artery stenting did not confer significant benefit over medical therapy with respect to preserving kidney function and preventing adverse cardiovascular events [5], [6], [7], [8]. However, revascularization still plays a substantial role in the treatment of RAS, depending on each patient’s clinical characteristics.
We herein describe a 78-year-old man with severe RAS who presented with heavy proteinuria and renal insufficiency and was treated successfully by angioplasty and repeated stenting.
Case report
A 78-year-old man known to have had hypertension for 5 years was admitted because of 2-month history of pitting edema in lower extremities. He had taken diuretics (furosemide) for a month, but still complained of edematous legs. He had been in good health until 2 months ago and had not taken any medication except for antihypertensive drug. The blood pressure (BP) was 125/70 mmHg on admission with amlodipine 5 mg orally once a day after switching from telmisartan 40 mg orally once a day about 2 weeks before admission. He did not show abdominal bruits and other physical signs suggesting infection. Laboratory evaluation showed serum creatinine (Cr) of 1.58 mg/dL, serum sodium of 142 mmol/L, serum potassium of 4.2 mmol/L, serum albumin of 3.8 g/dL, and total cholesterol of 192 mg/dL. Liver function profiles, uric acid, and serum electrolytes were within normal range. The number of white blood cells and the levels of C-reactive protein and erythrocyte sedimentation rate were also within normal range. His spot urine protein-to-Cr ratio (PCR) and spot urine albumin-to-Cr ratio (ACR) were 4.51 mg/mg and 3,943.8 μg/mg, respectively. Tests for hepatitis B surface antigen, antibody to hepatitis C virus, antinuclear antibodies, and antineutrophilic cytoplasmic antibodies were negative.
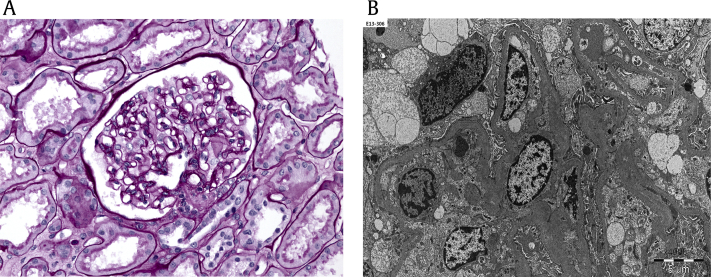
Renal ultrasonography showed right and left kidneys measuring 9.8 and 8.3 cm in length, respectively, with slightly increased cortical echogenicity. Percutaneous biopsy of the left kidney revealed mesangiopathic glomerulonephritis (Fig. 1A), with 30% of glomeruli showing global sclerosis. Immunofluorescence was negative, and electron microscopy was unremarkable (Fig. 1B). Heavy proteinuria could not be explained by the results of kidney biopsy. Small restrictions of the gamma globulin region were found at the serum electrophoresis, and an abnormal band was observed against anti-IgG and anti-kappa from serum immunofixation. However, bone marrow biopsy showed no definite evidence of clonality in plasma cells.
A week after resuming angiotensin receptor blocker (ARB) (losartan 50 mg orally once a day), spot urine PCR and ACR decreased from 4.51 to 2.30 mg/mg and from 3,943.8 to 1,887.1 μg/mg, respectively. However, renal function significantly deteriorated (increase in serum Cr from 1.73 to 2.75 mg/dL). ARB was discontinued because of rapidly progressive azotemia. Considering the size discrepancy of both kidneys and progressive azotemia by ARB, RAS was suspected. Therefore, renal artery computed tomography (CT) angiography and angiogram of renal artery were performed. The results showed severe stenosis of left main renal artery origin site (90%; Figs. 2A–B) and mild luminal narrowing of proximal right main renal artery (less than 30%). A stent was successfully inserted into the left renal artery (Fig. 2C). However, focal aortic dissection developed right after the intervention.
Three days after renal artery stenting, the spot urine PCR and ACR decreased from 2.30 to 0.55 mg/mg and from 1,887.1 to 77.8 μg/mg, respectively. However, serum Cr level increased to 2.84 mg/dL. Aorta noncontrast CT showed acute intramural hematoma at descending and abdominal aorta and localized dissection at the distal segment of abdominal aorta. Conservative management with tight control of BP was continued. Thoracoabdominal CT angiography taken 5 days later showed progression of the intramural hematoma of aorta. The diameter of the aorta was increased, and the aortic dissection extended from the origin of superior mesentery artery to right common iliac artery. The progressed aortic dissection partially blocked the entry of the stent originally inserted into left renal artery, but the left kidney still received blood flow from the true lumen of aorta (Fig. 3). Three days later, angiography was performed again because of persistent azotemia showing serum Cr higher than 2.0 mg/dL. Angiography showed that the entire orifice of left renal artery stent was in the false lumen because of progressed aortic dissection, so the left kidney was not receiving any blood flow from the true lumen (Fig. 4A). Another stent graft insertion into the original stent and balloon dilatation were therefore performed on left renal artery, restoring blood flow from the true lumen of the aorta (Fig. 4B). After intervention, serum Cr level decreased to 1.76 mg/dL, and the patient was discharged on aspirin and a β-blocker (atenolol). Spot urine PCR and ACR at discharge were 0.27 mg/mg and 156.6 μg/mg, respectively.
He has had stable renal function with serum Cr around 1.50 mg/dL and minimal microalbuminuria, and well-controlled BP was observed for more than 2 years after discharge.
Discussion
RAS is a common cause of curable hypertension and renal insufficiency [1], but has not been mentioned as a major cause of heavy proteinuria in previously reported reviews [2]. However, a few cases of nephrotic-range proteinuria in patients with renovascular disease have been reported, usually resulting from atherosclerosis, especially in the elderly [3], [4], [10], [11]. In some cases, massive proteinuria has been successfully treated with angiotensin-converting enzyme inhibitors (ACE-i) or ARB or by revascularization or removal of the affected kidney. In our case, an elderly patient presented with nephrotic-range proteinuria without typical signs of RAS, such as uncontrolled hypertension. Therefore, several work-ups including kidney biopsy and renal artery CT angiography were performed before the final diagnosis of RAS. Because the lesion of RAS was so tight, aortic dissection developed as a complication after renal artery stenting. Although we maintained appropriate conservative treatment, including tight control of BP, aortic dissection progressed, so we performed balloon angioplasty followed by insertion of another stent graft into the left renal artery. After interventions for left RAS, the patient's renal function and proteinuria improved markedly. We believe this case is valuable as an instructive case that appropriate intervention can be the treatment of choice for RAS presenting with nephrotic-range proteinuria in elderly patients.
Although the mechanism of nephrotic-range proteinuria in RAS remains unclear, increased glomerular membrane permeability caused by the effect of angiotensin II on the activation of the intrarenal renin–angiotensin system is probably involved. Renin activity was high in RAS patients with nephrotic-range proteinuria [3], [4], [12], and some cases showed improvements of proteinuria with ACE-i or ARB [3], [10]. This case could be evidence of proteinuria caused by activated rennin–angiotensin system because proteinuria was markedly improved by ARB treatment and revascularization of RAS. ACE-i and ARB are optimal antihypertensive choices for patients with atherosclerotic renovascular disease, especially those with coexisting coronary artery disease [13]. However, ACE-i and ARB in patients with RAS can cause renal dysfunction; therefore, careful monitoring of renal function is required, especially in patients with bilateral RAS [14].
Severe RAS usually leads to hyperfiltration and kidney ischemia, which may predispose an affected kidney to secondary forms of focal segmental glomerulosclerosis. In 1996, Thadhani et al [15] reported 24 cases of focal segmental glomerulosclerosis in patients aged older than 50 years and identified 7 patients with renovascular disease and substantial proteinuria. In our case, the pathologic finding of the left kidney was not typical in RAS, but mesangiopathic glomerulonephritis combined with RAS could cause the heavy proteinuria. And the marked decrement of proteinuria after treatment of RAS could tell that RAS was the main cause of nephrotic-range proteinuria.
Many researchers have examined treatments for atherosclerotic RAS [6], [7], [8]. In previous randomized trials, intervention including renal artery stenting was not superior to medical treatment in the aspect of preserving kidney function and controlling BP [6], [7]. Recently, a randomized trial including 947 patients with RAS compared 2 types of treatment: medical therapy plus renal artery stenting and medical therapy alone. That trial found no significant differences between the groups in cardiovascular or renal events, including myocardial infarction, stroke, hospitalization for congestive heart failure, progressive renal insufficiency, and the need for renal replacement therapy [8]. In a recent meta-analysis review, BP, renal function, and cardiovascular events were compared between medical therapy and revascularization, but analysis regarding proteinuria was not included [5]. Although no solid evidence supports that interventions for RAS are superior to medical treatment, renal prognosis can be definitely improved by intervention in some patients with RAS. In previously reported cases as well as our patient, angioplasty and subsequent stenting in RAS patients with nephrotic-range proteinuria improved renal outcome with an obvious decrease in proteinuria [11], [12].
In conclusion, RAS should be considered in the differential diagnosis of idiopathic nephrotic-range proteinuria, especially in elderly patients. Revascularization needs to be considered for the treatment of RAS with significant proteinuria, and careful monitoring for complications such as aortic dissection is needed.






 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















