| Kidney Res Clin Pract > Volume 35(1); 2016 > Article |
|
Abstract
Background
The aim of our study was to assess the relationship between soluble Klotho (s-Klotho) and carotid intimaãmedia thickness (CIMT) and left ventricular (LV) dysfunction in hemodialysis (HD) patients.
Methods
This is a cross-sectional study conducted on 88 patients with end-stage renal disease on regular HD. Serum levels of calcium, phosphorus, parathyroid hormone, and C-reactive proteinô were measured. The serum levels of s-Klotho and fibroblast growth factor-23 (FGF-23) were measured using an Enzyme linked immunosorbent assay (ELISA) kit. Echocardiography and measurement of CIMT were also conducted. The studied patients were divided according to the median s-Klotho level into 2 groups: patients with low s-Klotho (Group I) and patients with high s-Klotho (Group II).
Results
Mean value of s-Klotho was significantly low in HD patients compared to controls (Pô =ô 0.001), and mean value of FGF-23 was significantly high in HD patients compared to controls (Pô =ô 0.001). The mean values of parathyroid hormone, FGF-23, and phosphorus were significantly high in Group I compared to Group II, whereas the mean value of serum calcium was significantly low in Group I compared to Group II. The mean values of CIMT, LV mass (LVM), LVM index, and LV ejection fraction (LVEF) were high in Group I compared to Group II. Patients with low s-Klotho had significantly more coronary artery disease (CAD). In a regression analysis of s-Klotho with different markers of cardiovascular diseases, s-Klotho showed significant association with CIMT, LVEF, and CAD, but not with LVM and LVM index.
Conclusion
The present study showed thatô patients with a low s-Klotho were more often associated with increased CIMT, LV dysfunction, and CAD, and it seems thatô there was independent association between s-Klotho and CIMT, LVEF, and CAD.
Keywords
Cardiovascular diseases, End-stage renal disease, Fibroblast growth factor-23, Soluble KlothoChronic kidney disease (CKD) is associated with increased levels of parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF-23) and hypocalcemia, hyperphosphatemia, bone disease, vascular calcification, and cardiovascular morbidities collectively referred to as CKD-mineral and bone disorderô [1], [2], [3]. Recent reports suggested that increased levels of FGF-23 and decreased levels of soluble Klotho (s-Klotho) are common manifestations of CKD that develop earlier than increased levels of phosphate (Ps) or PTH [4], [5].
A transmembrane (TM) protein known as soluble öÝ-Klotho (s-Klotho) is primarily produced in the kidney distal tubular cells [6]. Soluble öÝ-Klotho acts as a coreceptor for the bone-derived protein FGF-23 [7], [8]. Regulation of both renal handling of Ps and renal synthesis of calcitriol needs a cofunction of both FGF-23 and TM-Klotho [9]. Soluble öÝ-Klotho is the circulating protein resulting from the shedding of the extracellular domain of TM-Klotho operated by 2 metalloproteinases of the A disintegrin and metalloproteinase domainãcontaining protein (ADAM) family: ADAM10 and ADAM17 [10]. In particular, s-Klotho inhibits the sodium-Ps cotransporter NaPi2a expression in the proximal tubules, thus generating a phosphaturic effect additive to and independent of FGF-23 [11], [12], [13], and activates the ion channel TRPV5 in the distal tubules, thus increasing tubular reabsorption of calcium (Ca) [14]. Therefore, öÝ-Klotho, with its TM and soluble forms, is deeply involved with the physiological regulation of mineral metabolism [15].
CKD is a common risk factor for cardiovascular diseases (CVD) such as coronary artery disease (CAD), cerebrovascular stroke, peripheral vascular disease, and heart failure [16]. Although part of CVD burden in patients with CKD is related to traditional risk factors, CKD-associated disturbance in CaãPs homeostasis plays a crucial role as well [2]. Recent studies showed thatô FGF-23 and its coreceptor s-Klotho have an important role in CaãPs homeostasis, and they could be the missing link in the detrimental relationship between CKD and CVD [17].
Given the strong cardioprotective effects of Klotho demonstrated in preclinical studies, the present study aimed to assess the association between s-Klotho and carotid intimaãmedia thickness (CIMT) and left ventricular (LV) dysfunction in patients with end-stage renal disease (ESRD) on regular hemodialysis (HD). It was hypothesized that low levels of s-Klotho are associated with a larger burden of CVD in these patients.
This is a cross-sectional study conducted on 88 patients with ESRDô on regular HD of at least 6-monthô duration. All patients have been attending the nephrology dialysis unit in the Theodor Bilharz Research Institute, Giza, Egypt. All patients received 3 sessions of HD/wk, each of 4-hourô duration using a polysulfone dialyzer (Fresenius, St. Wendel, Germany) with surface area of 1.4ã1.6. Patients with advanced congestive heart failure [defined clinically by elevated jugular venous pressure, ascites, peripheral edema, and shortness of breath at rest and LV ejection fraction (LVEF) of <30% in echocardiography requiring hospital-based support, a heart transplant, or palliative care according to guidelines of the American College of Cardiology/American Heart Association] [18], sepsis, or malignancy were excluded from the study. Informed written consent was obtained from all participants. The study protocol was approved by the institute ethics committee, and the study was performed in accordance with the Declaration of Helsinki.
The studied patients were divided according to the median s-Klotho level (cut point, 476ô pg/mL) into 2 groups: patients with low s-Klotho <476ô pg/mL (Group I, nô =ô 44) and patients with high s-Klotho >476ô pg/mL (Group II, nô =ô 44).
A control group consisting of 28 normal individuals was used to give our reference values for s-Klotho, FGF-23, CIMT, and echocardiographic findings. There were 17 men and 11 women with a mean age of 53.3ô ôÝô 16.2 years, with normal renal functionô and no evidence of acute or chronic underlying disease.
Each patient underwent a thorough history and clinical examination. Demographic characteristics and coexisting conditions such as atherosclerotic CAD diagnosed by electrocardiography and coronary angiography within 3 months of enrollment in the study were collected.
Fasting blood samples were collected from the patients before the HD session, immediately centrifuged, aliquoted in vials, and stored at ã60ô¯C untilô the time of analysis. Thawing the test samples was carried out at a low temperature by mixing them completely before measurement. Routine examinations included complete blood analysis, kidney function tests (serum urea, creatinine, sodium and potassium, and uric acid), random blood sugar, serum electrolytes, lipidô profile, and serum albumin. Serum levels of Ca, Ps, PTH, and C-reactive proteinô were measured.
The serum levels of s-Klotho were measured using an Enzyme linked immunosorbent assay (ELISA) system (Immuno-Biological Laboratories, Gunma, Japan) [19], and this assay detects circulating s-Klotho using 2 monoclonal antibodies that specifically recognize the extracellular domain of Klotho, with a lower limit of detection of 6.15ãô pg/mL and the intra-assay and interassay coefficients of variation of <10%. The serum levels of FGF-23 were measured using a commercial sandwich ELISA kit (Kainos Laboratories, Inc., Tokyo, Japan) [20]ô that uses a 2-site ELISA for the full-length molecule. Two specific murine monoclonal antibodies recognize the biologically active FGF-23, with a lower limit of detection of 3ô ãpg/mLô and interassay and intra-assay coefficients of variation of <5%.
Residual renal function (RRF) was estimated by calculating glomerular filtration rateô expressed in mL/min/1.73ãm2. Glomerular filtration rate was estimated as the mean of urea and creatinine clearance using 24-hourô urine collections and the mean of the post- and pre-HD plasma urea and creatinine. RRF was considered zero in patients with a urinary output <100ô mL/24ô hô [21]. Kt/V was used to assess dialysis adequacy [22].
After the HD session, each patient underwent echocardiography to determine LV mass (LVM), LVM index (LVMI), and LVEF. For determination of LVM, the Devereux formula was used [23]. LVM (g): 1.04 [(LVIDô +ô PWTô +ô IVST)3 ã LVID3] ã 14, where LVID indicates LV internal dimension, PWT indicates posterior wall thickness, and IVST indicates interventricular septal thickness. LVM was divided by body surface area to measure LVMI. According to the current guidelines, LV dysfunction was defined as an LVEFã < 45%.
After the HD session, each patient underwent ultrasonography of the carotid arteries using a high-resolution real-time scanner with a 7.5-MHz transducer [24]. The examination was done with the patient in the supine position, and the common carotid artery and carotid bifurcationô were scanned on both sides. The carotid artery was scanned in the longitudinal and transverse directions. The site of the most advanced atherosclerotic lesion that showed the greatest distance between the lumenãintima interface and the mediaãadventitia interface was the maximum intimaãmedia thickness (IMT) value. When plaque was detected on ultrasonography, it was observed as localized thickening rather than a circumferential change in the vessel wall. The greatest thickness of the intimaãmedia complex (including plaque) was used for the maximum IMT value. We identified patients having atherosclerosis based on atheromatous plaques of focal increases in IMT ãË 1.1ô mm in accordance with a prior study that showed the normal limit of IMT to be ãÊ1.0ô mm [25].
Data are presented as meansô ôÝô standard deviationsô or number (%). The 2-tailed Student's t test was used to compare the mean values of 2 groups (GraphPad Quick calc; Graphpad Software Inc., San Diego, CA, USA). Categorical data were compared using the chi-square test. The Spearman rank correlation test was used to analyze the correlations between s-Klotho levels and markers of mineral metabolism. Multivariate regression analysis (using MedCalc software; MedCalc software bvba, Acacialaan, Ostend, Belgium) was used to analyze the association between s-Klotho and markers of CVD, with a linear regression analysis model with unstandardized betas and a 95% confidence interval (CI) for CIMT, LVM, LVMI, and LVEF and a logistic regression analysis model was used for CAD. The linear regression analysis model was also used to determine the association between FGF-23 and CIMT, LVM, LVMI, and LVEF and the logistic regression analysis model was used for CAD. Statistical analyses of data were performed using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA). A Pô value< 0.05 was considered to be significant, and Pô <ô 0.01 was considered to be highly significant.
Demographic characteristics of HD patients and controls included in this study are shown in Tableô 1, where the mean age of studied patients was 58.6ô ôÝô 19.3ô years. Fifty-one (58.0%) patients were men and 37 (42.1%) were women. The most common causes of ESRD were diabetes mellitus, hypertension, and glomerulonephritis (Tableô 1).
Mean value of s-Klotho was significantly low in HD patients compared to the control group (477.9ô ôÝô 76.2 vs. 863.7ô ôÝô 261.8, Pô =ô 0.001), and mean value of FGF-23 was significantly high in HD patients compared to the control group (60.5ô ôÝô 17.6 vs. 35.8ô ôÝô 13.9, Pô =ô 0.001; Tableô 2).
Patients with a low s-Klotho (<476ô pg/mL) were older (63.2ô ôÝô 9.4 vs. 53.8ô ôÝô 16.2, Pô =ô 0.001) and had high FGF-23, PTH, Ps, and C-reactive protein and low Ca and RRF (Tableô 2) compared to Group II. There were no significant differences in the mean values of serum creatinine, lipidô profile, hemoglobin, serum glucose, serum albumin, serum uric acid, and Kt/V between the 2 groups (Tableô 2).
The mean values of serum levels of PTH, FGF-23, and Ps were significantly high in patients of Group I compared to patients of Group II, whereas the mean value of serum levels of Ca was significantly low in Group I compared to Group II (Tableô 2).
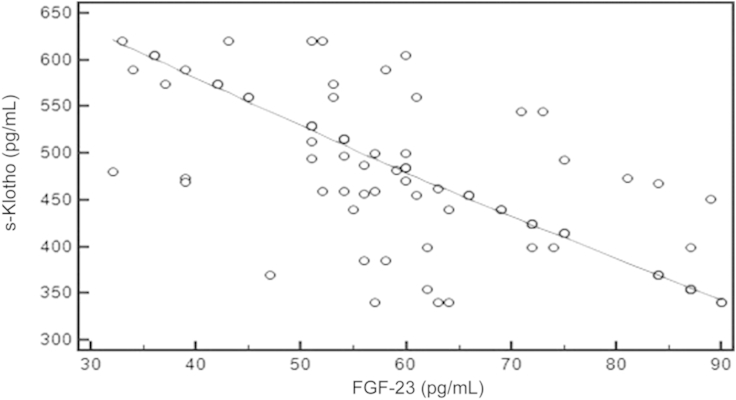
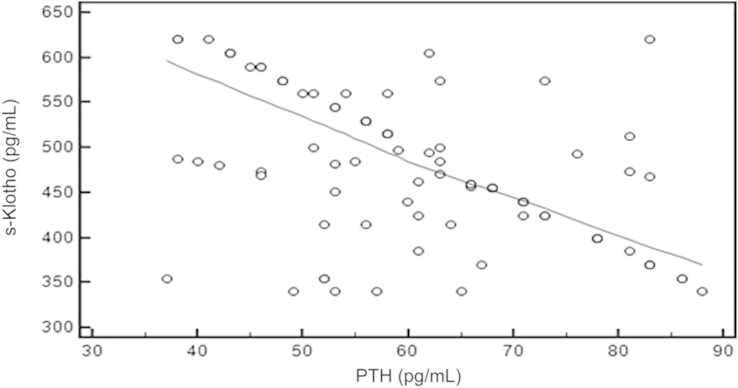
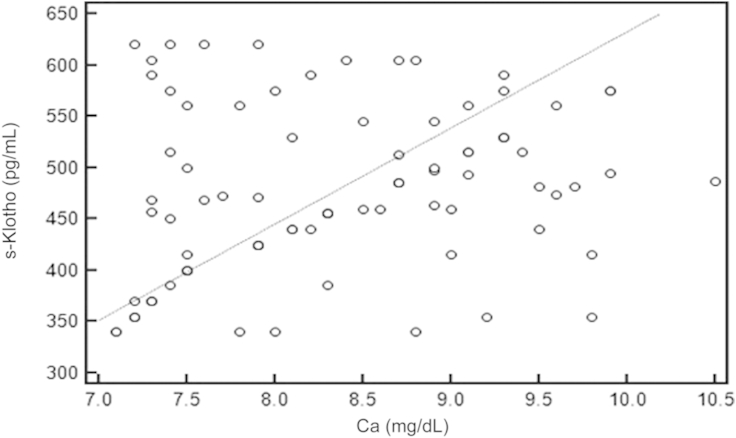
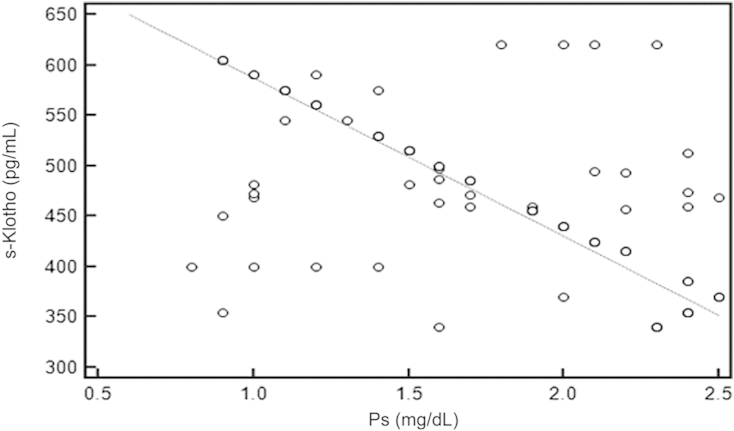
Using the Spearman rank correlation test, we found that there was significant negative correlation between s-Klotho and FGF-23 (rô =ô ã0.717, Pô =ô 0.001, 95% CIô for rô =ô ã0.806 to ã0.598; Fig.ô 1), PTH (rô =ô ã0.484, Pô =ô 0.001, 95% CI for rô =ô ã0.630 to ã0.306; Fig.ô 2), and Ps (rô =ô ã0.548, Pô =ô 0.001, 95% CI ã0.680 to ã0.383; Fig.ô 3), whereas there was significant positive correlation between s-Klotho and Ca (rô =ô 0.294, Pô =ô 0.005, 95% CI 0.0903ã0.474; Fig.ô 4).
The mean values of CIMT, LVM, LVMI, and LVEF were high in patients of Group I compared to patients of Group II. The prevalence of CAD was higher among patients with low s-Klotho [36 (81.1%) vs. 22 (50.0%), Pô =ô 0.004; Table 3].
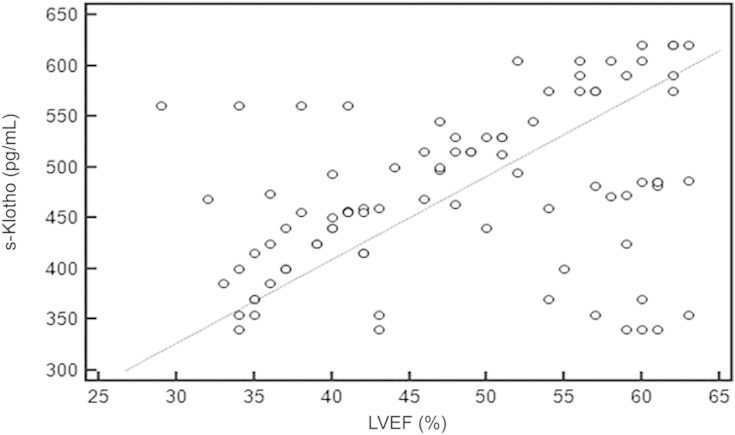
Using the Spearman rank correlation test, we found that there was significant positive correlation between s-Klotho and LVEF (rô =ô 0.392, 95% CI 0.199ã0.556, Pô =ô 0.001; Fig.ô 5).
Tableô 4 showsô the results of the multivariate regression analysis of s-Klotho with CIMT, LVM, LVMI, LVEF, and CAD as different markers for CVD. After adjustments for age, gender, mean blood pressure, and duration of HD, s-Klotho showed significant association with CIMT, LVEF, and CAD, but not with LVM and LVMI.
Using the Spearman rank correlation test, we found that there was positive correlation between FGF-23 and Ps (rô =ô 0.497, Pô =ô 0.001, 95% CI 0.321ã0.640) and PTH (rô =ô 0.405, Pô =ô 0.001, 95% CI 0.214ã0.567), whereas there was negative correlation between FGF-23 and Ca (rô =ô ã0.237, Pô =ô 0.026, 95% CI ã0.426 to ã0.029).
Tableô 4 shows the results of multivariate regression models of FGF-23, total cholesterol, triglycerides, and blood glucose with CIMT, LVM, LVMI, LVEF, and CAD as different markers for CVD. After adjustments for age, gender, mean blood pressure, and duration of HD, FGF-23, total cholesterol, triglycerides, and blood glucose were not independently associated with CIMT, LVM, LVMI, LVEF, and CAD.
In the present study, we measured the s-Klotho levels and determined the association between s-Klotho levels and markers of mineral metabolism (FGF-23, PTH, Ca, and Ps)ô and CVD (CIMT, CAD, LVM, LVMI, and LVEF) in patients with ESRD on regular HD. Our study showed thatô there was significant negative correlation between s-Klotho levels and serum levels of FGF-23, PTH, and Psô and positive correlation between s-Klotho and serum levels of Ca. In addition, the present study showed that lower levels of s-Klotho are significantly associated with signs of CVD such as increased CIMT, more CAD, and LV dysfunction.
Our study showed that serum levels of s-Klotho were significantly decreased in patients with ESRD on regular HD compared to the control group, as previously reported in CKD patients [26] and patients on HD [27], [28], [29]. It has been reported that s-Klotho levels are severely reduced in patients with CKD compared to control subjects [30]. However, it appears that s-Klotho levels are not completely depleted, even in patients with ESRD on HD [27], [28], [29]. This finding suggests that in humans, a basal level of s-Klotho may be produced from other organs than the kidneys, such as the brain and parathyroid glands, as previously reported in mice [15], [31], [32]. Recent studies revealed thatô s-Klotho has been decreased in the early stage of CKD, even before rising of Ps or PTH and emerged as a powerful player in CaãPs homeostasis that is thought to contribute to the high burden of CVD in CKD patients [5], [17]. This study showed thatô patients with a low s-Klotho (<476ô pg/mL) had a high age and low RRF compared to Group II, and there was no significant differences in the mean values of Kt/V between the 2 groups. Our results are in agreement with the results of Lindberg etô al [28], who reported that patients with high s-Klotho were more with good RRF, and Sawires etô al [29], who reported that there was no significant correlation between s-Klotho and Kt/V and there was significant negative correlation between s-Klotho and age.
The present study showed thatô patients with a low s-Klothoô were more often associated with CIMT, LV dysfunction, and CAD, and it seems thatô there was independent association between s-Klotho and CIMT, LVEF, and CAD using multivariate regression analysis. Our results are in agreement with results of Kitagawa etô al [33]ô who reported that the serum Klotho level was found to significantly correlate with markers of CKD-mineral and bone disorder and is an independent biomarker of arterial stiffness in patients with CKD. In contrary to our results, Buiten etô al [34]ô reported that s-Klotho was not independently associated with CVD in dialysis patients and did not support a direct cardioprotective effect of s-Klotho.
Many clinical studies have suggested that s-Klotho exerts strong cardioprotective effects. For instance, s-Klotho has been shown to protect against vascular calcifications in rodent models of CKD, whereas in humans without CKD, higher s-Klotho levels have been related to a lower incidence of mortality and CVD [28], [35], [36], [37]. Moreover, low s-Klotho levels have been associated with increased arterial stiffness in CKD patients [33]. Further support for a direct role of Klotho in vascular homeostasis comes from inô vitro studies showing endogenous expression of s-Klotho in human vascular smooth muscle cellsô [38]. Interestingly, inhibition of s-Klotho expression in aortic vascular smooth muscle cells resulted in accelerated calcification of these cells [38]. However, the exact role of s-Klotho in the progression of CVD in dialysis patients remains to be elucidated.
There many reports that CVD starts to develop in early stages of CKD and that s-Klotho starts to decrease also in early stages of CKD. Therefore, patients with ESRD on regular HD have been exposed to low s-Klotho levels for a prolonged period, predisposing them to vascular calcifications and atherosclerosis.
The role of s-Klotho in the development of atherosclerotic disease in dialysis patients might be overshadowed by the large amount of other pathophysiological stimuli for CVD prevalent in these patients, such as obesity, diabetes, dyslipidemia, and hypertension. This might explain the disparity between data found in rodents with only Klotho deficiency and patients suffering from a wide variety of comorbidities. However, our study showed thatô there were no significant differences in the mean values of lipidô profile, blood glucose, mean blood pressure, body mass index, and duration of dialysis between the 2 groups. Using multivariate regression analysis, we found that there was no independent association between FGF-23, blood glucose, total cholesterol, and triglycerides and CVD markers. These findings may explain thatô s-Klotho seems to be independently associated with CIMT, LVEF, and CAD as markers of CVD.
In our study, s-Klotho is significantly correlated with markers of mineral metabolism, which is consistent with many studies in CKD patients [5], [33]ô that reported that s-Klotho correlated negatively with PTH and Ps and positively with Ca, whereas no correlation was found between s-Klotho and 1,25 dihydroxycholecalciferolô and fractional excretion of Ca. Also our study is consistent with studies in HD patients [34]ô that reported that the serum s-Klotho level was significantly associated with a lower plasma 25 hydroxy vitamin D [25(OH)D] and lower PTH [29].
In addition, in our study, FGF-23 was significantly associated with markers of mineral metabolism, and FGF-23 was not independently associated with markers of CVD. Our results are consistent with studies in HD patients [34]ô that reported that FGF-23 showed a strong positive association with Ps and PTH and FGF-23 was not independently associated with CVD. In addition, our results are comparable with the results from studies in CKD patients, where FGF-23 levels correlated positively with PTHô and Ps and negatively with 1,25 dihydroxycholecalciferol and no correlation existed with Ca and fractional excretion of Ca [5].
This study has some limitations. We studied only a small sampleô of patients. Data on dietary Ca, Ps, and medicationô intake, which may affect the levels of serum Ps, Ca, s-Klotho, and FGF-23 were not collected. Ps and PTH were not statistically analyzed as risk factors for CVD.
In conclusion, the present study showed thatô patients with a low s-Klotho were more often associated with increased CIMT, LV dysfunction, and CAD, and it seems thatô there was an independent association between s-Klotho and CIMT, LVEF, and CAD.
References
1. Moe S., Drû¥eke T., Cunningham J., Goodman W., Martin K., Olgaard K., Ott S., Sprague S., Lameire N., Eknoyan G.; Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69:2006;1945ã1953.


2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 76(Supplô 113):2009;S1ãS130.
3. Moe S.M., Drueke T.. Improving global outcomes in mineral and bone disorders. Clin J Am Soc Nephrol 3(Suppl 3):2008;S127ãS130.



4. Isakova T., Wahl P., Vargas G.S., Gutiûˋrrez O.M., Scialla J., Xie H., Appleby D., Nessel L., Bellovich K., Chen J., Hamm L., Gadegbeku C., Horwitz E., Townsend R.R., Anderson C.A., Lash J.P., Hsu C.Y., Leonard M.B., Wolf M.. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79:2011;1370ã1378.



5. Rotondi S., Pasquali M., Tartaglione L., Muci M.L., Mandanici G., Leonangeli C., Sales S., Farcomeni A., Mazzaferro S.. Soluble öÝ-klotho serum levels in chronic kidney disease. Int J Endocrinol 2015:2015 872193.Published online 2015 [Abstract].




6. Mian I.S.. Sequence, structural, functional, and phylogenetic analyses of three glycosidase families. Blood Cells Mol Dis 24:1998;83ã100.


7. Kurosu H., Ogawa Y., Miyoshi M., Yamamoto M., Nandi A., Rosenblatt K.P., Baum M.G., Schiavi S., Hu M.C., Moe O.W., Kuro-o M.. Regulation of fibroblast growth factor-23 signaling by klotho. Jô Biol Chem 281:2006;6120ã6123.

8. Urakawa I., Yamazaki Y., Shimada T., Iijima K., Hasegawa H., Okawa K., Fujita T., Fukumoto S., Yamashita T.. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:2006;770ã774.


9. Liu S., Tang W., Zhou J., Stubbs J.R., Luo Q., Pi M., Quarles L.D.. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. Jô Am Soc Nephrol 17:2006;1305ã1315.

10. Chen C.D., Podvin S., Gillespie E., Leeman S.E., Abraham C.R.. Insulin stimulates the cleavage and release of the extracellular domain of klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA 104:2007;19796ã19801.



11. Hu M.C., Kuro-o M., Moe O.W.. Klotho and chronic kidney disease. Contrib Nephrol 180:2013;47ã63.



12. Hu M.C., Kuro-o M., Moe O.W.. Renal and extrarenal actions of klotho. Semin Nephrol 33:2013;118ã129.



13. Hu M.C., Shi M., Zhang J., Pastor J., Nakatani T., Lanske B., Razzaque M.S., Rosenblatt K.P., Baum M.G., Kuro-o M., Moe O.W.. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24:2010;3438ã3450.



14. Cha S.K., Ortega B., Kurosu H., Rosenblatt K.P., Kuro-O M., Huang C.L.. Removal of sialic acid involving klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105:2008;9805ã9810.



16. Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y.. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. Nô Engl J Med 351:2004;1296ã1305.

17. Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., Fujita T., Nakahara K., Fukumoto S., Yamashita T.. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. Jô Bone Miner Res 19:2004;429ã435.

18. Hunt S.A., Abraham W.T., Chin M.H., Feldman A.M., Francis G.S., Ganiats T.G., Jessup M., Konstam M.A., Mancini D.M., Michl K., Oates J.A., Rahko P.S., Silver M.A., Stevenson L.W., Yancy C.W., Antman E.M., Smith S.C. Jr., Adams C.D., Anderson J.L., Faxon D.P., Fuster V., Halperin J.L., Hiratzka L.F., Jacobs A.K., Nishimura R., Ornato J.P., Page R.L., Riegel B.; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 112:2005 e154ãe235. Published online 2005 Sep 13. doi:10.1161/CIRCULATIONAHA.105.167586.

19. Yamazaki Y., Imura A., Urakawa I., Shimada T., Murakami J., Aono Y., Hasegawa H., Yamashita T., Nakatani K., Saito Y., Okamoto N., Kurumatani N., Namba N., Kitaoka T., Ozono K., Sakai T., Hataya H., Ichikawa S., Imel E.A., Econs M.J., Nabeshima Y.. Establishment of sandwich ELISA for soluble alpha-klotho measurement: age-dependent change of soluble alpha-klotho levels in healthy subjects. Biochem Biophys Res Commun 398:2010;513ã518.



20. Imel E.A., Peacock M., Pitukcheewanont P., Heller H.J., Ward L.M., Shulman D., Kassem M., Rackoff P., Zimering M., Dalkin A., Drobny E., Colussi G., Shaker J.L., Hoogendoorn E.H., Hui S.L., Econs M.J.. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. Jô Clin Endocrinol Metab 91:2006;2055ã2061.

21. European Best Practice Guidelines Expert Group on Hemodialysis; European Renal Association. Section I. Measurement of renal function, when to refer and when to start dialysis. Nephrol Dial Transplant 17:2002;7ã9.
22. Daugirdas J.T.. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. Jô Am Soc Nephrol 4:1993;1205ã1213.

23. Devereux R.B., Reichek N.. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 55:1977;613ã618.


24. Nakamura A., Shikata K., Hiramatsu M., Nakatou T., Kitamura T., Wada J., Itoshima T., Makino H.. Serum interleukin-18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Care 28:2005;2890ã2895.


25. Handa N., Matsumoto M., Maeda H., Hougaku H., Ogawa S., Fukunaga R., Yoneda S., Kimura K., Kamada T.. Ultrasonic evaluation of early carotid atherosclerosis. Stroke 21:1990;1567ã1572.


26. Shimamura Y., Hamada K., Inoue K., Ogata K., Ishihara M., Kagawa T., Inoue M., Fujimoto S., Ikebe M., Yuasa K., Yamanaka S., Sugiura T., Terada Y.. Serum levels of soluble secreted alpha-klotho are decreased in the early stages of CKD, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 16:2012;722ã729.


27. Yokoyama K., Imura A., Ohkido I., Maruyama Y., Yamazaki Y., Hasegawa H., Urae J., Sekino H., Nabeshima Y., Hosoya T.. Serum soluble alpha-klotho in hemodialysis patients. Clin Nephrol 77:2012;347ã351.

28. Lindberg K., Amin R., Moe O.W., Hu M.C., Erben R.G., ûstman Wernerson A., Lanske B., Olauson H., Larsson T.E.. The kidney is the principal organ mediating klotho effects. Jô Am Soc Nephrol 25:2014;2169ã2175.

29. Sawires H.K., Essam R.M., Morgan M.F., Mahmoud R.A.. Serum klotho: relation to fibroblast growth factor-23 and other regulators of phosphate metabolism in children with chronic kidney disease. Nephron 129:2015;293ã299.


30. Koh N., Fujimori T., Nishiguchi S., Tamori A., Shiomi S., Nakatani T., Sugimura K., Kishimoto T., Kinoshita S., Kuroki T., Nabeshima Y.. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280:2001;1015ã1020.


31. Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., Iwasaki H., Iida A., Shiraki-Iida T., Nishikawa S., Nagai R., Nabeshima Y.I.. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:1997;45ã51.


32. John G.B., Cheng C.Y., Kuro-o M.. Role of klotho in aging, phosphate metabolism, and CKD. Am J Kidney Dis 58:2011;127ã134.



33. Kitagawa M., Sugiyama H., Morinaga H., Inoue T., Takiue K., Ogawa A., Yamanari T., Kikumoto Y., Uchida H.A., Kitamura S., Maeshima Y., Nakamura K., Ito H., Makino H.. Aô decreased level of serum soluble klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One 8:2013 e56695.Published online 2013 Feb 19. doi:10.1371/journal.pone.0056695.



34. Buiten M.S., de Bie M.K., Bouma-de Krijger A., van Dam B., Dekker F.W., Jukema J.W., Rabelink T.J., Rotmans J.I.. Soluble klotho is not independently associated with cardiovascular disease in a population of dialysis patients. BMC Nephrol 15:2014;197.



35. Hu M.C., Shiizaki K., Kuro-o M., Moe O.W.. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75:2013;503ã533.



36. Semba R.D., Cappola A.R., Sun K., Bandinelli S., Dalal M., Crasto C., Guralnik J.M., Ferrucci L.. Plasma klotho and cardiovascular disease in adults. Jô Am Geriatr Soc 59:2011;1596ã1601.

Figureô 1
Correlation coefficient between the serum level of s-Klothoô and FGF-23.rô =ô ã0.717, 95% confidence interval, ã0.806 to ã0.598, Pô =ô 0.001.
FGF-23, fibroblast growth factor-23; s-Klotho, soluble Klotho.
Figureô 2
Correlation coefficient between the serum level of s-Klothoô and PTH.rô =ô ã0.484, 95% confidence interval ã0.630 to ã0.306, Pô =ô 0.001.
PTH, parathyroid hormone; s-Klotho, soluble Klotho.
Figureô 3
Correlation coefficient between the serum levels of s-Klothoô and Ca.rô =ô 0.294, 95% confidence interval 0.0903ã0.474, Pô =ô 0.005.
Ca, calcium; s-Klotho, soluble Klotho.
Figureô 4
Correlation coefficient between the serum levels of s-Klothoô and Ps.rô =ô ã0.548, 95% confidence interval ã0.680 to ã0.383, Pô =ô 0.001.
Ps, phosphate; s-Klotho, soluble Klotho.
Figureô 5
Correlation coefficient between s-Klothoô and LVEF.rô =ô 0.392, 95% confidence interval 0.199ã0.556, Pô =ô 0.001.
LVEF, left ventricular ejection fraction; s-Klotho, soluble Klotho.
Tableô 1
Demographic characteristics of the studied patients and control group
APKD, adult polycystic kidney disease; BMI, body mass index; BP, blood pressure; CIN, chronic interstitial nephritis; DBP, diastolic blood pressure; DM, diabetes mellitus; ESRD, end-stage renal disease; GN, glomerulonephritis; HTN, hypertension; SBP, systolic blood pressure; SLE, systemic lupus erythematosus.
Tableô 2
Laboratory parameters of the studied patients and control group
Data are presented as mean ôÝ SD. Bl., blood; Ca, calcium; C-RP, C-reactive protein; FGF-23, fibroblast growth factor-23; Hb, hemoglobin; LDL-C, low-density lipoprotein cholesterol; Ps,ô phosphorus; PTH, parathyroid hormone; RRF, residual renal function; S., serum; sCr, serum creatinine; s-Klotho, soluble Klotho; T., total; TG, triglyceride.
Tableô 3
Echocardiographic findings, CIMT, and CAD of the studied patients and control group
Tableô 4
Multivariate linear regression analysis of the predictive factors for CIMT, LVM, LVMI, LVEF, and CAD



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















