| Kidney Res Clin Pract > Volume 34(4); 2015 > Article |
|
Abstract
We report the first case of Ramsay Hunt syndrome (RHS) diagnosed after kidney transplantation in Korea. RHS is a disease caused by latent varicella-zoster characterized to involve geniculate ganglion of the seventh cranial nerve. Patients who have undergone kidney transplantation can be easily affected by viral infections because of their immune-compromised status. A 35-year-old man with hypertensive end-stage renal disease underwent kidney transplantation. Two months after surgery, the recipient was diagnosed with RHS and treated with antivirals and steroids. However, after using the antiviral agents for the recommended duration, facial paralysis occurred as a new presentation and he required further treatment. Otalgia and periauricular vesicles improved, but the facial palsy remained.
Keywords
Kidney transplantation, Ramsay Hunt syndrome, Varicella-zoster virus, Facial palsyLatent infection of varicella-zoster virus (VZV) is an issue in managing kidney transplant recipients. Because these patients are using various combinations of immunosuppressive agents, incidence of severe forms of viral infection is reported relatively high compared with that of normal immune status. Ramsay Hunt syndrome (RHS), also known as herpes oticus, is a rare manifestation of latent VZV infection and was first described by Ramsey Hunt [1]. This syndrome is caused by the reactivation of latent VZV in geniculate ganglion of the seventh cranial nerve and is characterized by otalgia, vesicle, and facial palsy of the affected side. RHS occurs more commonly in immunosuppressed individuals, such as kidney transplant recipients [2], [3], but it has not been reported previously in Korea. Here, we describe the first case of RHS diagnosed and treated with antiviral agents and glucocorticoids in a 35-year-old kidney transplant recipient in Korea.
A 35-year-old kidney transplant recipient presented to the nephrology outpatient clinic because of left otalgia and periauricular vesicles. He underwent deceased-donor kidney transplantation because of hypertensive end-stage renal disease about 2 months before the first symptoms appeared and had been using immunosuppressive medications. His regimen included tacrolimus 3 mg, mycophenolic acid 720 mg twice daily, and prednisolone 20 mg once daily. At the time of transplantation, we checked his viral markers as a protocol, and the patient did not recall his vaccination status. He underwent emergency surgery before we checked the final result of the viral markers. Later, we found out that his serum VZV immunoglobulin (Ig)-G was positive and IgM was negative (Fig. 1).
Initial vital signs were stable. In addition to pain and vesicular rash around the left ear, he complained of numbness of the left half of his face, but neurologic examination revealed no abnormal findings. Routine laboratory tests were conducted and revealed a serum creatinine level of 1.9 mg/dL. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease equation, and the value was 48 mL/min. The tacrolimus level was measured immediately before ingestion, and the result was 4.8 ng/mL, which was slightly below the target range.
In addition, serum VZV IgG/IgM was measured, and serum VZV polymerase chain reaction (PCR) was performed (Fig. 2). However, his symptoms were sufficiently clear to make a diagnosis of RHS with or without serologic confirmation of VZV infection, and we were not reluctant to administer intravenous (i.v.) antiviral agents and steroids in this case. Acyclovir was administered at a dose of 500 mg i.v. (5 mg/kg, 95 kg) twice daily for 5 days. The preferred dose of acyclovir in patients with normal renal function is 5–12.4 mg/kg twice daily. However, considering his renal impairment, dose reduction was performed. To administer steroids via the i.v. route and considering an anti-inflammatory effect, methylprednisolone was chosen. Methylprednisolone 62.5 mg was given i.v. and then tapered out. Although serum VZV IgM was negative, a positive result was obtained on serum VZV PCR, so etiologic evidence was confirmed. As symptoms improved, the patient was discharged with oral antivirals and prednisolone. He was treated with famciclovir 750 mg once daily for an additional 10 days along with a maintenance dose of prednisolone (20 mg once daily). However, 3 days after cessation of famciclovir, he returned to the hospital because of a sudden onset of a severe spinning-type dizziness associated with nausea and left facial palsy. Neurologic examinations were conducted again, and nystagmus was observed at the primary position. When we evoked the nystagmus using the Dix–Hallpike test, left torsional nystagmus was observed in both eyes. In addition, on testing his facial paralysis, forehead blinking was decreased. These observations indicated that facial palsy was a result of peripheral nerve abnormality, but brain magnetic resonance imaging (MRI) was performed to exclude central vertigo and to evaluate the swelling of the facial nerve. In both T1- and T2-weighted images, microbleeding ∼9 mm in size was noted in the right pons. However, judging from its signal intensity, it was a chronic lesion rather than an acute lesion, and current dizziness was irrelevant. No swelling of the left facial nerve was seen. We restarted oral famciclovir 750 mg once daily with oral methylprednisolone for 7 days. This time we used oral methylprednisolone instead of i.v. because the patient complained of the i.v. catheter. The starting dose of oral methylprednisolone was 64 mg which was tapered every 2 days with a reduction of 16 mg each time. In addition, we lowered the dose of mycophenolate mofetil from 720 mg twice daily to 540 mg twice daily. We maintained the dose of tacrolimus because its serum level was at the lower limit of the therapeutic range. In addition to medical treatment, we encouraged self-facial massage and consulted for rehabilitation.
The patient was discharged with a slight improvement of facial paralysis. Electromyography was conducted 2 weeks after the onset to evaluate his facial motor and sensory nerves. This examination showed no abnormal spontaneous activity and neuropathy action potentials, but reduced recruitment of left frontalis, orbicularis oculi, nasalis, and orbicularis oris was noted. Otalgia and periauricular vesicles improved, but left facial palsy still remained. After 1 month, his facial palsy showed improvement.
RHS is a disease caused by latent VSV infection involving the geniculate ganglion of the seventh cranial nerve [1]. Kidney transplant patients can be easily affected by viruses and show severe extents of disease because of immunosuppressive medications that must be taken [2]. The incidence of VZV infection is ∼11.2% within 4 years after kidney transplantation [4], [5] but that of RHS has not been reported probably due to the rarity of the disease.
Measuring serum VZV IgG/IgM is essential before the transplant. A vaccination for seronegative candidates should be considered if the transplant is elective.
The patient described here showed the RHS symptom triad, and virologic confirmation was performed using VZV PCR. The disease was diagnosed easily, but treatment was challenging because he received a kidney from a donor with a high creatinine level (1.8 mg/dL). We considered his estimated glomerular filtration rate and had to reduce the dose of acyclovir. Typical brain MRI findings of RHS include enhancement of the affected facial nerve as a consequence of destruction of the blood–brain barrier [6]. In this case, facial nerve enhancement was not observed, but evidence of chronic pontine microbleeding was noted. We performed a brain MRI 14 days after the onset because his clinical signs and symptoms were compatible with typical peripheral vertigo. Because we used an antiviral agent and steroid for the 14 days, facial nerve swelling might have been improved at the time of the MRI. Preexisting bleeding in the central nervous system was thought to be a consequence of hypertension. RHS is treated with steroids to reduce the inflammation, and a starting dose of 50–60 mg of prednisolone is commonly used along with an i.v. antiviral agent to suppress virus replication [7], [8], [9]. Acyclovir is believed to reduce nerve damage, so its clinical usefulness is widely accepted [10]. In addition, adjunctive therapies, such as facial massage and acupuncture, can be useful, but there have been no systematic studies to evaluate the effectiveness of these treatments. However, unlike RHS in normal populations, there are several additional considerations in treating RHS in kidney transplant recipients. Posttransplant care for kidney recipients is difficult because care is required in the application of medications to protect renal function.
Table 1 presents a literature review of RHS after kidney transplantation. Initial treatment using i.v. acyclovir and steroids (either i.v. or oral) was the same for all cases. All four cases of patients showed improvement after using acyclovir and steroids. However, time to achieve remission varied. It ranged from 10 days to 10 months.
Maintaining the balance between rejection and the opportunistic infection is always troublesome after organ transplantation. In addition, in treating RHS, methylprednisolone is used for its anti-inflammatory effects, but steroid use can also suppress the host immunity simultaneously, so there is increased risk of virus infection. The duration of steroid use and the reduction of immunosuppressants are dependent on the clinician׳s decision. This case is worth reporting because it is the first case of RHS diagnosed after kidney transplantation in Korea.
References
1. Hunt J.R.. On herpetic inflammation of the geniculate ganglion: a new syndrome and its complications. J Nerv Ment Dis 34:1907;73–96.

2. Mortada RA, El Fakih RO, Assi M. Unusual presentation of Ramsay Hunt syndrome in renal transplant patients: case report and literature review. Transpl Infect Dis 11:2009;72–74.


3. Otsuki K, Kenmochi T, Maruyama M, Akutsu N, Iwashita C, Ito T, Matsumoto I, Asano T. A case of Ramsay Hunt syndrome in living-kidney transplant recipient. Transplant Proc 44:2012;307–308.


4. Arness T, Pedersen R, Dierkhising R, Kremers W, Patel R. Varicella zoster virus–associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis 10:2008;260–268.


5. Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 20:2005;748–753.

6. Suzuki F, Furuta Y, Ohtani F, Fukuda S, Inuyama Y. Herpes virus reactivation and gadolinium-enhanced magnetic resonance imaging in patients with facial palsy. Otol Neurotol 22:2001;549–553.


7. Park JM, Yu SJ, Park AR, Lee SM. Treatment of Ramsay Hunt syndrome that is mistaken for trigeminal herpes zoster. Korean J Pain 21:2008;237–240.

8. McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry 63:1997;321–326.

9. Kinishi M, Amatsu M, Mohri M, Saito M, Hasegawa T, Haseqawa S. Acyclovir improves recovery rate of facial nerve palsy in Ramsay Hunt syndrome. Auris Nasus Larynx 28:2001;223–226.


10. Peterslund NA, Seyer-Hansen K, Ipsen J, Esmann V, Schonheyder H, Juhl H. Acyclovir in herpes zoster. Lancet 2:1981;827–830.


Figure 1
Clinical course of the patient. Bid, twice a day; Day, days from the onset of otalgia; IV, intravenously; MPA, mycophenoleic acid; MPDL, methylprednisolone; PCR, polymerase chain reaction; qd, once a day; VZV, varicella-zoster virus.
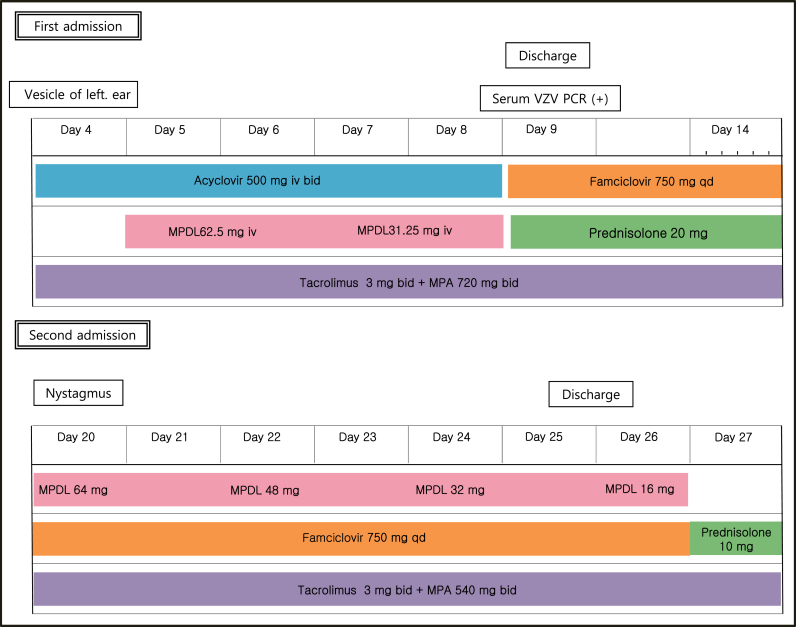
Table 1
Literature review of Ramsay Hunt syndrome after kidney transplantation
| No. | Age/sex | 1. Onset after KT | Immunosuppressant | Treatment |
|---|---|---|---|---|
| 2. Donor | ||||
| 3. Cause of ESRD | ||||
| 1 [3] | 36/F | l. l mo | l. MMF 1,500 mg | IV Acyclovir (3 mg/kg, 3 times/d) Reduction of the MMF dose from 1,500 mg/d to 1,000 mg/d PO prednisolone 50mg for 3 days |
| 2. Living donor | 2. Tacrolimus 6 mg | |||
| 3. Polycystic kidney disease | ||||
| 2 [2] | 41/M | l. 4 yr | l. MMF 1,500 mg | IV Acyclovir (10 mg/kg, 3 times/d) Myringectomy with tube placement in the left middle ear Oral prednisone |
| 2. Living donor | 2. Tacrolimus 2.6 mg bid | |||
| 3. IgAN | ||||
| Valacyclovir 1,000 mg 3 times/d | ||||
| 3 [11] | 35/M | l. 8 mo | l. MPA 1,440 mg | IV Acyclovir (250 mg, 3 times/d) |
| 2. Not mentioned | 2. Tacrolimus 2.5 mg/d | IV Methylprednisolone (100 mg, 1 time/d) | ||
| 3. Unknown etiology | Reduction of the MMF dose from l,440 mg/d to 720 mg/d | |||
| 4 [12] | 27/M | l. 18 mo | l. MMF 2,000 mg | IV Acyclovir 10 mg/kg/d, 3 times/d PO Acyclovir (400 mg. 5 times/d) Oral prednisolone dosage was tapered |
| 2. Living donor | 2. Cyclosporin | |||
| 3. Not mentioned |
- TOOLS
-
METRICS

- Related articles
-
Necessity of induction agent modification for old age kidney transplant recipients
C4d Puzzle in ABO-incompatible kidney transplantation2024 January;43(1)
Malakoplakia in the recipient’s native kidney after kidney transplantation2023 January;42(1)
The effect of periodontitis on recipient outcomes after kidney transplantation2022 January;41(1)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















