A case of biopsy-proven chronic kidney disease on progression from acute phosphate nephropathy
Article information
Abstract
Acute phosphate nephropathy (APhN) following oral sodium phosphate solution (OSP) ingestion as a bowel purgative has been frequently reported. It was recently suggested that APhN could progress to chronic kidney disease (CKD) and a history of APhN might be considered as one of the causes of CKD. However, there are few reports proving APhN as a cause of CKD. Here, we report a case of APhN that progressed to CKD, as proven by renal biopsy.
Introduction
Colonoscopy is a widespread diagnostic tool for the evaluation of colonic diseases. Bowel preparation is an important factor in obtaining an accurate diagnosis. Patients use a purgative the day before the procedure to improve the accuracy of the diagnosis. Oral sodium phosphate (OSP) and polyethylene glycol (PEG) solutions are commonly used for bowel preparation because of their ease of use and their safety profile [1].
OSP has an advantage over PEG in terms of a lower fluid volume to be ingested (two bottles of 45 mL each). Initially, the adverse effects of this agent were unremarkable, especially in patients with normal kidney function. However, because of cases of OSP-induced acute renal failure, the safety of OSP has been questioned [2]. Furthermore, it was noted that acute renal failure can progress to chronic kidney disease (CKD) [3]. Here, we report a case of CKD caused by acute phosphate nephropathy (APhN) following use of OSP as a bowel purgative.
Case report
A 51-year-old woman was admitted to Inha University Hospital for evaluation of renal failure that was recently found at a local clinic.
She had been diagnosed with essential hypertension 3 years previously, which was managed with hydrochlorothiazide 12.5 mg and candesartan 16 mg once a day. Her other previous medical history was unremarkable. She took ibuprofen 400 mg on the initial day of menstruation on a monthly basis for 30 years to relieve abdominal pain. Seven months previously, she underwent a health screening examination. Bowel preparation involved ingestion of OSP (Phospho Soda, Fleet, USA). After 8 hours of NPO, she visited the health promotion center at 09:00 hours. Blood and urine were sampled for laboratory examination. Then, esophagogastroduodenoscopy (EGD) and colonoscopy were performed under anesthesia using intravenous midazolam 5 mg. Serum blood urea nitrogen (BUN; 8.8 mg/dL), creatinine (0.86 mg/dL), calcium (9.0 mg/dL), phosphate (4.7 mg/dL), and albumin (4.3 g/dL)) were all normal at that time. EGD and colonoscopy findings were normal. However, the patient suffered from persistent nausea and anorexia after the screening examination.
Her physical examination showed no abnormalities. Blood pressure was 130/70 mmHg, pulse rate was 72/minute, and body temperature was 37 °C.
Blood tests yielded the following results: hemoglobin 8.3 g/dL, platelets 350,000/μL, white blood cells 8170/μL, BUN 42.5 mg/dL, creatinine 2.95 mg/dL, calcium 8.5 mg/dL, phosphate 3.7 mg/dL, total protein 7.9 g/dL, albumin 4.0 g/dL, glucose 102 mg/dL, sodium 140 mEq/L, potassium 4.6 mEq/L, chloride 103 mEq/L, and total CO2 21.4 mEq/L. C3 was 134 mg/dL, C4 was 34 mg/dL, and antinuclear antibody, anti-neutrophil cytoplasm antibody, cryoglobulin, RA factor, anti-ds DNA antibody, and anti-GBM antibody tests were all negative. Serum iron was 40 μg/dL, total iron-binding capacity was 303 μg/dL, ferritin was 28.7 μg/mL, and stool occult blood was negative according to EGD. Serum and urine electrophoresis analyses were normal. Urine specific gravity was 1.009 and protein and blood were negative. The 24-hour urine volume was 3700 mL, with protein of 595 mg and creatinine of 1.0 g.
A bone marrow biopsy showed no evidence of multiple myeloma or other diseases. Abdominal ultrasonography showed an increased renal cortical echo-texture. However, renal contour and size were normal (right 11.9 cm, left 11.3 cm, long axis; Fig. 1) and there was no evidence of a renal stone or hydronephrosis.
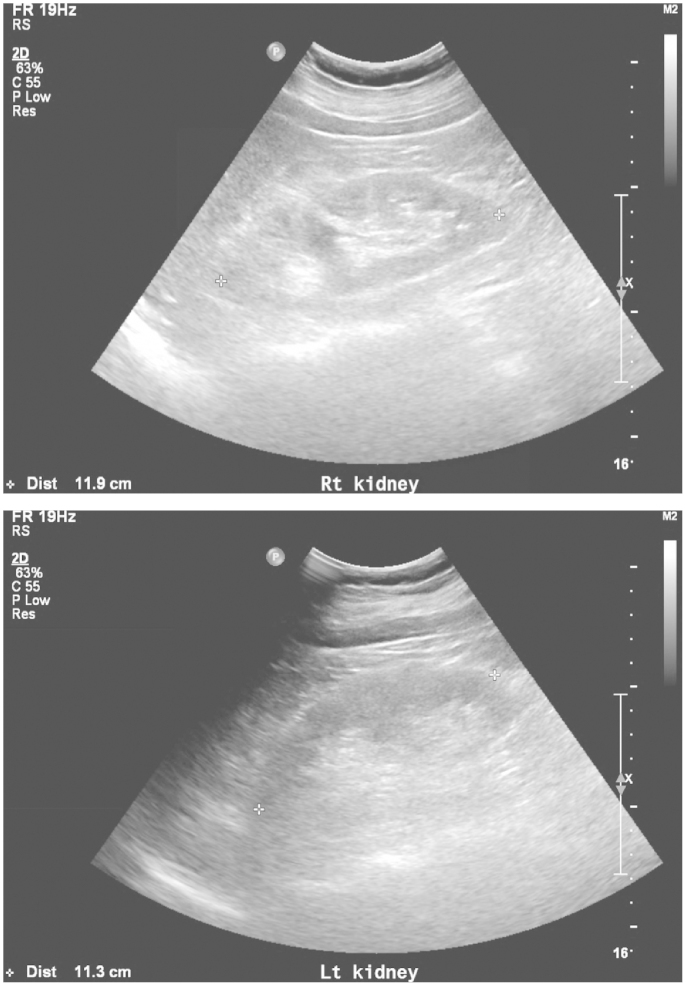
Abdominal ultrasonography showed increased renal cortical echo-texture, but the renal contour and size were normal (right, 11.9 cm; left, 11.3 cm, long axis).
The patient was hydrated with 1–2 L of normal saline for 10 day. However, her serum creatinine level remained elevated (2.74 mg/dL). To find the cause of renal failure, a kidney biopsy was performed on Day 10. Pathologic analysis of the kidney biopsy showed tubular atrophy or dilation (Fig. 2A). Multifocal scattered calcifications were present within the tubules, mainly in the distal tubules (Fig. 2B). The tubular epithelial cells were simplified and showed degenerative and regenerative changes. The interstitium showed a moderate degree of fibrosis and inflammatory cell infiltration, mainly of lymphocytes and some neutrophils (Fig. 3). The tubular calcifications did not polarize and were positive to von Kossa stain (Fig. 3), which confirmed their composition as calcium phosphate. The glomeruli appeared mildly enlarged and there were no capillary wall changes. Immunohistochemistry was negative for immunoglobulins and for complement factors.

The kidney biopsy showed tubular atrophies or dilation (Masson's trichrome stain, 100× (a) and scattered calcifications within the tubules (hematoxylin and eosin stain, 100× (b).
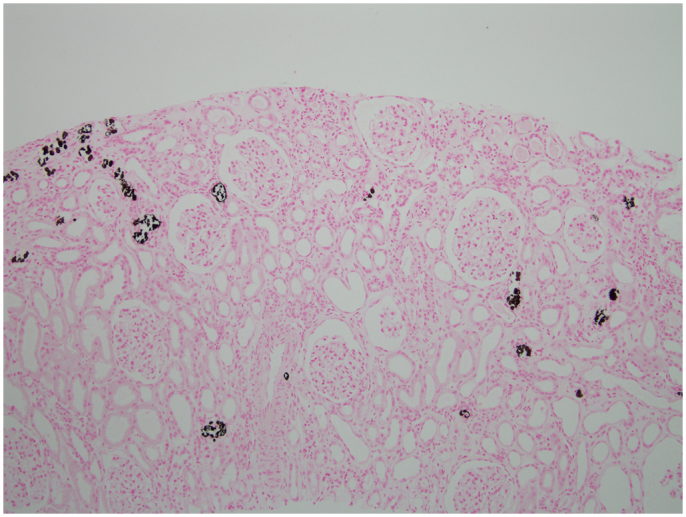
A positive histochemical reaction with von Kossa stain confirms that the tubular concretions are composed of calcium phosphate (100×).
After kidney biopsy, a further examination was performed to rule out other causes of nephrocalcinosis. Serum intact parathyroid hormone (iPTH) decreased from an initial level of 186 pg/mL to 54.6 pg/mL after 2 months. Results for 24-hour urinary analytes were as follows: sodium, 98 mEq/day (normal range 40–220 mEq/day); uric acid, 425 mg/day (250–750 mg/day); citric acid, 16 mg/day (320–1240 mg/day); oxalate, 3.36 mg/day (16.20–53.30 mg/day); calcium, 35.0 mg/day (70.0–180.0 mg/day); and creatinine, 0.9 g/day (1.0–2.0 g/day).
Supportive treatment was given and the patient's kidney function remained unchanged 3 months later, with a creatinine level of 2.21 mg/dL.
Discussion
This case shows that exposure to OSP as a bowel purgative is associated with the development of APhN in patients with risk factors and can progress to CKD. Furthermore, in CKD patients with unknown cause, clinicians should consider that use of an OSP bowel purgative may be one of the possible causes of the development of CKD.
OSP preparations are osmotic purgatives that obligate water excretion into the intestinal lumen to maintain its isotonicity with plasma [2]. Retention of water in the bowel lumen results in peristalsis and colonic evacuation. The high osmolarity of OSP is due to its high sodium and phosphate content [2]. Each 45-mL dose of Phospho Soda contains 5 g of sodium and 17.8 g of phosphate, yielding a solution with osmolarity of 16,622 mOsm/L [2]. Various complications, including hyperphosphatemia, hypocalcemia, hypernatremia, hypokalemia, metabolic acidosis, and acute kidney injury (AKI), have been reported in association with OSP ingestion [4]. Hyperphosphatemia is considered to be a major cause of OSP-induced AKI. Therefore, Desmeules et al. used the term APhN for AKI following OSP use as a bowel purgative [5]. The incidence has been reported as 1.29–5.0% [6], [7].
Calcium phosphate deposition in renal tubules and interstitium appears to be a mechanism underlying APhN and has been demonstrated histopathologically using von Kossa stain [7]. Nephrocalcinosis occurs when calcium precipitates in conjunction with either oxalate or phosphate [2]. The phosphates in calcium phosphate deposits are detected in paraffin sections using von Kossa stain [2], which involves a precipitation reaction in which silver ions react with phosphate to yield a black product. This stain does not detect pure calcium oxalate [2]. In the case of hyperparathyroidism, calcium salts are typically found along medullary tubular basement membranes, as concretions within tubules, and in the interstitium [4]. By contrast, the calcium phosphate deposits seen in APhN are found primarily in the tubular lumen and the cytoplasm of tubular epithelial cells, with rare interstitial deposits as well [8]. Hypercalciuria and hyperoxaluria are also risk factors for nephrocalcinosis [2]. In our case, the elevated serum iPTH level seemed to be due to CKD, and biopsies in such cases show calcium phosphate deposits, primarily in the tubular lumen. Our case did not show hypercalcuria, hyperoxaluria or a history of herb ingestion. Among patients with CKD of unknown cause, it has been reported that Chinese herb nephropathy may be an etiologic agent, in which progressive tubulointerstitial nephritis and development of end-stage renal disease within 1–2 years are characteristics [9], [10]. Our patient did not have a history of herb ingestion.
Following intake of a large amount of phosphate, proximal tubular phosphate reabsorption is decreased, leading to a rapid increase in delivery to the distal tubule [11]. Diarrhea-induced hypovolemia leads to avid salt and water reabsorption in both the proximal tubule and the descending limb of Henle's loop, which is relatively impermeable to calcium and phosphate [12]. The net effect is a decrease in phosphate reabsorption in the proximal tubule, leading to a marked increase in calcium–phosphorus products within the lumen of the distal tubule [8]. Hypovolemia-associated tubular injury may also precondition the distal tubular epithelium, leading to surface expression of hyaluronan and osteopontin, which in turn creates a suitable environment for adherence of calcium phosphate crystals [12]. A direct toxic effect of hyperphosphatemia on renal tubules is proposed as one of the mechanisms of renal failure after OSP use. In a rat model of APhN involving intravenous administration of phosphorus, Zarger et al. observed vacuolization of tubular cells without calcium phosphate deposition in renal biopsies [16].
Old age, female gender, liver disease, renal disease, decreased bowel motility, hypertension, diabetes, and medications contributing to renal hypoperfusion, such as diuretics, angiotensin-converting enzyme inhibitors and angiotensin (AT)–II receptor blockers, could predispose to this disorder [11]. In our case, the patient had risk factors of female gender, hypertension, and AT-II receptor blocker use.
The development of CKD in APhN following the use of OSP has been reported [12]. The pathophysiology has not been elucidated yet, but it appears to be associated with activation of the innate immune system in mediating the pro-inflammatory effects of tissue crystal deposition [2]. Pattern recognition receptors, such as the Toll-like receptor (TLR), mediate monosodium urate monohydrate-dependent nitric oxide and IL-1β release from chondrocytes and macrophages, respectively [2]. Given the ability of specific TLRs to recognize calcium crystals and the expression of a variety of TLRs in adult kidney, it is possible that intraluminal calcium phosphate crystal formation after OSP is recognized by specific epithelial TLRs, with subsequent activation of the innate immune response [13]. Persistence of these crystals could result in chronic inflammation with extracellular matrix deposition and interstitial fibrosis [14].
We speculate that iron deficiency anemia in our case was caused by a decreased iron intake due to persistent anorexia.
There are only a few reports in terms of CKD after APhN following OSP ingestion as a bowel purgative so far in Korea. Kim et al. reported a case of APhN following OSP use as a bowel purgative in a 66-year-old female [15]. We report a case of APhN that progressed to CKD, as proven by renal biopsy, in a female patient of normal renal function prior to colonoscopy.
Conflict of interest
None declared.