Genetic predisposition of donors affects the allograft outcome in kidney transplantation: Single-nucleotide polymorphism of aquaporin-11
Article information
Abstract
Background
Aquaporin-11 (AQP11) is a novel member of the aquaporin family. Disruption of the murine Aqp11 gene causes severe proximal tubular injury and renal failure. The rs2276415 (G>A) single-nucleotide polymorphism in the human AQP11 gene results in glycine to serine substitution in a functionally important domain. In this study, the role of the genetic predispositions of AQP11 rs2276415 (G>A) on renal allograft outcomes was evaluated.
Methods
A total of 198 pairs of donors and recipients were enrolled in this study. Long-term graft survival was traced and clinical parameters that could have influenced graft outcome were collected through the electronic medical record system.
Results
The genotype distribution and allele frequency of rs2276415 polymorphism were not different between donors and recipients. Despite similar allele frequencies between donors and recipients, the minor allele rs2276415 (GA+AA) of AQP11 from the donors, but not from the recipients, had a harmful effect on the graft survival compared with the wild-type donor (GG; P=0.029). This association was significant after adjusting for several risk factors including age, sex, human leukocyte antigen mismatch, donor type, hypertension, and diabetes mellitus (P=0.032).
Conclusion
A donor-derived, not recipient-derived, genetic AQP11 polymorphism has different effects on graft outcome. Thus, the genetic influence from donors should be carefully considered for proper management of allografts after kidney transplantation.
Introduction
Aquaporins (AQPs) are a family of membrane water channel proteins found throughout the animal and plant kingdoms [1]. Currently, 13 aquaporin family members have been identified in mammals. Aquaporin-11 (AQP11) is one of the newly described members, and its function is still not clearly understood.
Northern blot analyses showed that AQP11 expression was highest in the testis, and moderate in the kidney, liver, and brain. In the kidney, AQP11 is most abundantly expressed in the proximal tubule. A previously conducted study showed that AQP11-null mice died due to advanced renal failure with polycystic kidneys [2]. Vacuoles originated prior to cysts from the endoplasmic reticulum (ER), and AQP11 was considered to be important for ER homeostasis. These results imply that AQP11 is important in kidney development and function.
An unpublished study reported that rs2276415 (G>A) single-nucleotide polymorphism (SNP) in the human AQP11 gene, which results in Gly102Ser substitution in a functionally important domain, is associated with increased risk of acute kidney injury and chronic kidney disease [3]. These results indicate that AQP11 insufficiency predisposed the kidney to renal dysfunction.
Various immunological and nonimmunological determinants may affect the outcome of a renal allograft in kidney transplantation. The genetic interactions between donors and recipients are also an important issue. In this study, it is assumed that the graft outcome is dependent not only on the recipient’s response but also on the responses of the graft as an active participant. Therefore, it was hypothesized that genetic variation in the AQP11 proteins of both donors and recipients might affect long-term graft survival in kidney transplantation. In this study, the role of genetic predisposition of AQP11 rs2276415 (G>A) on renal allograft outcomes is analyzed.
Methods
Study population
A total of 198 pairs of Korean recipients and donors who were followed up for at least 1 year were recruited for this study. They had received kidney transplants at Seoul National University Hospital, Seoul, Korea between 1987 and 2008. Whole-blood samples from the recipients and their donors were collected as follows: 96 samples from donors and recipients; 25 samples from recipients only, and 77 samples from donors only.
The research protocol used for this study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. C-1205-061-410). All clinical investigations were conducted according to the guideline in the Declaration of Helsinki.
Medical record of recipients based on the electronic medical record system was reviewed. Clinical parameters that could have influenced graft outcome were collected, which included recipient’s sex and age at transplantation, history of hypertension, diabetes mellitus, and donor type. Estimated glomerular filtration rate of donor was calculated by the Modification of Diet in Renal Disease equation [4]. Delayed graft function was defined as need for dialysis in the 1st week after transplantation. Cytomegalovirus (CMV) and BK virus infections were evaluated with CMV antigenemia and plasma BK virus polymerase chain reaction, respectively.
The primary outcome of this study was graft loss defined as graft dysfunction that necessitated renal replacement therapy after transplantation.
Genotyping
DNA was extracted from whole blood, and genotyping for AQP11 rs2276415 (C_12041092_1) was carried out by the TaqMan SNP genotyping assays (7900HT fast real-time PCR system; Applied Biosystems, Foster city, CA, USA).
A different fluorescence label (6-carboxyfluorescein for wild type and 4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein for mutant) was used to label the 5′ segment of allelic probes. The TaqMan minor groove binder probe sequence was as follows: AQP11 rs2276415 5′-GGTGGGCACGTCCAGCAACCCGTGC[A/G]GCGTGATGATGCAGATGATGCTGGG-3′. Reaction mixtures consisted of a 1.0 µL 10× AmpliTaq buffer, 1.0 µL deoxynucleotide triphosphates (2.5mM each), 0.2 µL forward primer (20 pmol/µL), 0.2 µL reverse primer (20 pmol/µL), 1.0 µL genomic DNA (50 ng/µL), and 0.15 µL iMax II Taq polymerase. Polymerase chain reactions were carried out under the following conditions: 5 minutes at 94°C (1 cycle); 30 seconds at 94°C; 30 seconds at 56°C (35 cycles); 50 seconds at 72°C; and 7 minutes at 72°C (1 cycle).
Tissue immunohistochemical staining and analysis
To evaluate the AQP11 expression in kidney tissue according to the SNP, 15 kidney biopsy samples that showed the least pathology among recipients were selected. For immunohistochemical study, paraffin-embedded graft blocks of recipients were cut into 4 μm slices. For deparaffinization and hydration, xylene and ethanol were used. Endogenous streptavidin activity was blocked by 0.3% hydrogen peroxide (H2O2). To examine the expression of human AQP11, deparaffinized sections were stained with rabbit anti-AQP11 antibody (Novus Biologicals, Littleton, CO, USA). Antigen retrieval was carried out by heating paraffin-embedded sections in 10% citrate buffer in a microwave oven three times (each of 5 minutes duration). streptavidin and 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louise, MO, USA) were used for immunohistochemical detection. For each kidney sample, three fields were viewed at 200× magnification under a light microscope. Sections were then counterstained with Mayer's hematoxylin and examined by light microscopy. All morphometric parameters were determined using a microscope coupled to a computerized morphometry system (Qwin3; Leica, Rijswijk, The Netherlands).
Statistical analysis
SPSS for Windows package 12.0 K (SPSS Inc., Chicago, IL, USA) was used for all analyses and calculations. Student t test was used for continuous variables, and the results were presented as mean±standard deviation. The Chi-square test was used for categorical variables. Graft survival was analyzed using the Kaplan–Meier method, and comparison among groups was performed by the log-rank test. Values of P<0.05 were considered statistically significant.
Results
Frequency of genetic variants of AQP11
The genotype frequencies of the renal transplant recipients and donors did not show significant deviation from the Hardy–Weinberg equilibrium (P>0.05). The genotype and allele frequencies of the AQP11 rs2276415 SNP were not significantly different between the recipients and donors as a control (P=0.491; Table 1).
Baseline characteristics
Among the 198 pairs of recipients and donors, AQP11 rs2276415 SNP assays were conducted on 173 samples from donors and on 121 samples from recipients. Baseline characteristics were compared according to the presence of “A” allele in donors (GG vs. GA+AA). There were no statistically significant differences between the two groups in all evaluated variables, including sex, age, human leukocyte antigen (HLA) mismatch, and comorbidities (Table 2).
AQP11 expression according to AQP11 rs2276415 polymorphism
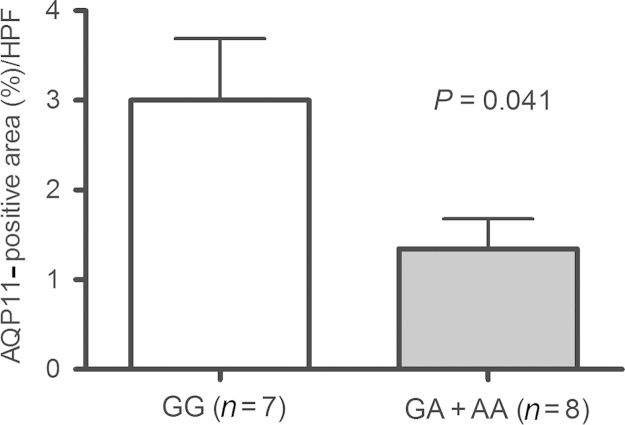
Among the 15 selected biopsy samples, eight were diagnosed as no abnormality or having borderline changes and the remaining samples were classified as diverse. After AQP11 immunohistochemical analysis, the most prominent staining of AQP11 was detected in the proximal tubule (Fig. 1). When the expression of AQP11 was assessed by the computerized morphometry system in 15 recipients based on donor AQP11 genotypes, AQP11 expression was significantly higher in the graft from donors with the wild-type genotype (GG), compared with the GA or AA genotype (Fig. 2, P=0.041).

Examples of immunohistochemical staining of AQP11 in the kidney. AQP11 was most prominently detected in the proximal tubule. The level of AQP11 expression was different according to the rs2276415 (G>A) polymorphism. (A) GG genotype and (B) AA genotype. AQP11, aquaporin-11.
Long-term graft survival and AQP11 rs2276415 polymorphism
Among the 198 pairs, graft loss occurred in 35 cases during the average follow-up period of 84.1 months. When survival analysis was performed using the Kaplan–Meier method, recipients receiving the kidney with heterozygous genotype (GA) or homozygous variant allele (AA) from donors showed poor graft survival as compared with recipients who received kidneys with the wild-type genotype (GG) in the log-rank test (Fig. 3B, P=0.029). Recipients’ polymorphism did not affect graft survival (Fig. 3A, P=0.382).
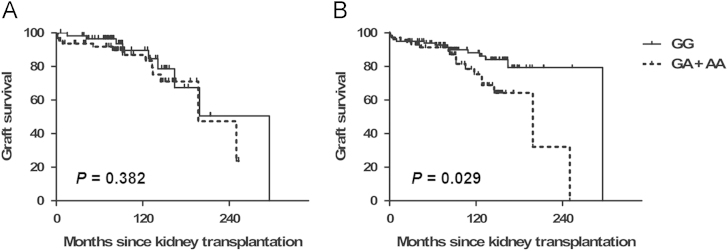
Graft survival according to theAQP11rs2276415 (G>A) genotypes. (A) Recipients and (B) donors. The minor allele rs2276415 (GA+AA) of AQP11 from the donors, but not from the recipients, has a harmful effect on the graft survival compared with the wild-type donor (GG). P value was taken from the log-rank test. AQP11, aquaporin-11.
We also evaluated the effect of recipients’ polymorphism among those receiving the kidney with GG genotype donors. Among 101 patients who received a kidney with GG genotype donors, 34 recipients had GG genotype, 15 had GA or AA, and 49 cases did not have the recipients’ sample. When survival analysis was performed using the Kaplan–Meier method, the graft survival was still not different between the GG and GA+AA genotype recipients (P=0.136).
For further evaluation of donors’ polymorphism, univariate and multivariate Cox regression analyses were performed after adjusting for several factors. Unadjusted Cox regression analysis revealed that individuals carrying the GA+AA genotype had an increased hazard ratio of graft loss [GA+AA vs. GG genotype, hazard ratio=2.20, 95% confidence interval (CI) 1.06–4.55; P=0.034]. This association was statistically significant after adjusting for several risk factors including age, sex, HLA mismatch, donor type, hypertension, and diabetes mellitus (hazard ratio=5.06, 95% CI 1.15–22.4; P=0.032).
Discussion
In this study, the influence of the human AQP11 rs2276415 polymorphism on allograft survival after kidney transplantation was investigated. The results indicated that donors’ AQP11 rs2276415 polymorphism is associated with an increased risk of graft loss. The influence remained evident after adjusting for possible confounding factors such as age, sex, HLA mismatch, donor type, hypertension, and diabetes mellitus.
AQP11 was revealed with the completion of the human genome project and was first reported in 2000 [2]. Even after 15 years, its biological role has not been fully evaluated. In the kidney, AQP11 is known to be localized intracellularly in the proximal tubule. AQP11-null mice were born normally but died prior to weaning due to advanced renal failure with polycystic kidneys [2]. Mutations of PKD1 and PKD2 genes, which encode polycystin proteins, are well evaluated as a common cause of autosomal dominant polycystic kidney disease and relations between polycystins and cilia function have been described [5]. According to a recent study, absence of AQP11 impaired glycosylation processing and caused aberrant membrane trafficking of polycystin-1 and abnormal ciliary length of proximal tubules, which could be a key mechanism of cystogenesis in AQP11-null mice [6]. AQP11 is surely an emerging molecule in the field of kidney research.
AQPs are characterized by forming tetramers, and each monomer contains a channel [7]. The topological model includes six transmembrane domains connected to each other by five loops (A–E). Previous AQPs have two highly conserved, short sequences named NPA (asparagine–proline–alanine) boxes, which are in loops B and E. These conserved signature motifs play an important role in water permeation through AQPs. However, AQP11 has a unique amino acid sequence pattern that includes an NPC (asparagine–proline–cysteine) motif corresponding to the N-terminal NPA signature motif of conventional AQPs [8].
Through several studies, one important site at the ninth residue downstream of the C-terminal NPA motif of AQP11 has been found. Tchekneva et al [9] reported that the chemical mutagen n-ethyl-n-nitrosourea induces an amino acid substitution (Cys227Ser) in mouse AQP11. This mutant mouse exhibited a lethal phenotype that resembles the one present in the AQP11-null mice. A subsequent study revealed that Cys227Ser mutation in AQP11 developed proximal tubule-specific mitochondrial injury by glucose-induced oxidative stress [10]. Takahashi et al [11] also emphasized the importance of the specific amino acid substitution in their recent report. They observed that Cys227Ser or Cys227Ala mutation interferes with the formation of three-dimensional multimeric structure in AQP11.
The N-terminal NPC motif of AQP11 is also essential for its function [12]. Site-directed mutation at the NPC motif (Cys101Ala) reduced oligomerization of AQP11 and significantly lowered osmotic water permeability. The currently studied AQP11 rs2276415 (G>A), which results in Gly102Ser substitution, is located just next to the NPC motif.
One unpublished study showed that patients with AQP11 rs2276415 (G>A) SNP are at a higher risk of acute kidney injury and developing chronic kidney disease [3]. Although this SNP has not been thoroughly studied, its location implies its importance. In this study, we investigated the AQP11 expression by tissue immunohistochemical staining. The GA and AA groups showed significantly weak staining as compared with the wild-type genotype (GG) group. Therefore, this SNP just next to the NPC motif seems to be morphologically important and might change the AQP11 expression and function.
Then, why does the AQP11 expression affect renal graft function? The possible mechanism seems to be related to ER homeostasis maintained by AQP11. In previous studies, AQP11 was repeatedly proved to localize to proximal tubule cells, especially in the ER, where the unfolded polypeptide chains are folded and mature into proteins [2,6]. During the flux of unfolded polypeptide chains, cells control the protein-folding capacity of ER according to the physiologic need and also maintain the quality of proteins. These regulatory processes are called “ER homeostasis” and the imbalanced situation, that is, the accumulation of unfolded and misfolded proteins, is referred to “ER stress” [13]. In a previous study, microarray analyses and histochemical staining revealed that genes and molecules related to ER stress and apoptosis were upregulated in AQP11 knockout mice [14]. The authors suggested that maintaining ER homeostasis might be an important physiologic function of AQP11 as a water channel because the intracellular environment is changing dynamically in the proximal tubules with a large amount of water reabsorption. According to another recent study, the aberrant post-translational modification of polycystin-1, which is responsible for ER function, was revealed as a possible mechanism of cystogenesis in polycystic kidneys of AQP11 knockout mice [6]. Based on these results, AQP11 seems to be important for maintaining ER homeostasis in the proximal tubule.
In solid organ transplantation, there are challenges that may induce ER stress such as ischemia-reperfusion (I/R) injury, chronic ischemia, calcineurin inhibitors, and inflammation [15]. Regarding I/R injury, adenosine triphosphate deficiency induced by hypoxia and nutrient deprivation during ischemia induces protein misfolding [16]. In kidney I/R models, several pathways related to ER stress induced by I/R have been studied [17,18]. In addition, considerable evidence is available to suggest that calcineurin inhibitor, the mainstay immunosuppressant in renal transplantation, may cause ER stress [19,20]. In the course of these challenges, low expression of AQP11 might negatively affect graft survival.
Our study has several limitations. First, samples of only 198 pairs of kidney transplantation were available and included in the analysis. This might cause selection bias. Second, the biopsy slides had diverse diagnosis, which could affect AQP11 expression directly.
In summary, a donor-derived, not recipient-derived, genetic AQP11 rs2276415 (G>A) polymorphism has different effects on graft outcome. Thus, the genetic influence from donors should be carefully considered for proper management of allografts after kidney transplantation. Further investigation is needed to clarify the role of AQP11 in kidney.
Conflict of interest
All authors declare no conflicts of interest.
References
Acknowledgments
This study was supported by a National Research Foundation grant funded by the Korean Government (Grant No. 800-20120365).