Introduction
Vascular complications after renal transplantation occur frequently and can lead to the deterioration of renal function, graft loss, and even death [1]. Iliac vein stenosis is a rare complication after renal transplantation, with only a few cases reported to date [2], [3], [4]. Here, we describe a case of iliac vein stenosis that developed 16 years after transplantation in a 56-year-old renal transplant recipient. Written informed consent was obtained from the study participant for publication of this case report and any accompanying images.
Case report
A 56-year-old male with a 16-year history of renal transplantation was admitted for a painless right lower limb edema that had developed 1 month earlier. The leg swelling waxed and waned over the course of each day without continuous leg pain, chest discomfort, or dyspnea.
The clinical workup revealed pitting edema and a loss of arterial pulsation in the right leg without discoloration or focal tenderness. Laboratory examinations showed normal prothrombin time and activated partial thromboplastin time, with a d-dimer concentration of 0.52┬Ā┬Ąg/mL fibrinogen-equivalent units (FEU; reference concentration <0.5┬Ā┬Ąg/mL FEU). His serum creatinine concentration was measured at 1.34┬Āmg/dL, and his estimated glomerular filtration rate (GFR) according to the Modification of Diet in Renal Disease (MDRD) formula was 55┬ĀmL/min/1.73┬Ām2, which were slightly changed from his baseline serum creatinine concentration (1.1┬Āmg/dL) and estimated GFR (69┬ĀmL/min/1.73┬Ām2).
Radionuclide venography demonstrated a delay in upward drainage at the level of the right common iliac vein, and collateral flow into the left iliac vein. Computed tomography (CT) angiography revealed severe stenosis in the right common iliac vein, with kinking at the bifurcation of the right common iliac artery. Venous flow from the transplanted renal vein drained through the right external iliac vein, subsequently collecting in the regional collateral and left iliac veins. Neither extrinsic compression due to perivascular fibrosis nor peritransplant fluid collection, such as hematoma and lymphocele, was detected. CT also excluded thrombosis in the deep venous system and renal transplant vein.
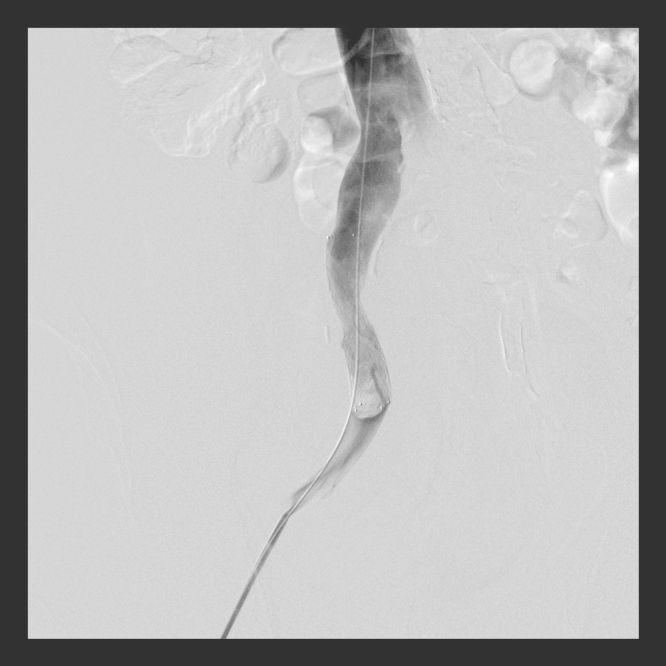
Pelvic venography after puncture of the right groin with 7F sheaths confirmed near-complete occlusion of the right common iliac vein and collateral venous flow (Fig. 1). Thrombolysis or thrombectomy was not necessary because there were no apparent intraluminal clots. A self-expanding 14 mm├Ś6 cm Zilver Vena stent (Cook Medical, Bloomington, IN, USA) was deployed without using angioplasty balloons, across the area of stenosis. Follow-up venograms demonstrated a brisk flow through the right common iliac vein without collateral filling and a patent renal transplant vein (Fig. 2).
The right lower limb edema rapidly resolved after the intervention, and the patientŌĆÖs serum creatinine concentration stabilized at 0.91┬Āmg/dL 3 days after venous stenting. The patient was free of procedure-related complications and was discharged in a stable condition with only aspirin therapy. After 6 months of follow-up, the patient remained asymptomatic with a functioning graft and the absence of lower limb edema.
According to the patientŌĆÖs medical records, he had received a living related donor kidney transplant 16 years previously for end-stage renal disease secondary to hypertensive nephropathy. Prior to transplantation, the patient had undergone hemodialysis for 14 years via a left-arm arteriovenous fistula, with no prior insertion of femoral dialysis catheters. The transplant kidney had two arteries and two veins, which were terminolaterally anastomosed onto the corresponding right external iliac vasculature. His postoperative course was uneventful, with neither surgical complications nor graft dysfunction requiring dialysis. The patient was discharged with a well-functioning graft and a serum creatinine concentration of 1.1 mg/dL.
Five years after renal transplantation, the patient was admitted to hospital because of an abrupt onset of graft tenderness, decreased urine output, and elevated level of serum creatinine (5.1┬Āmg/dL). Prerenal causes of acute kidney injury were less likely because the fractional excretion of filtered sodium was 1.2%. A Doppler ultrasonography (US) examination of the graft showed that echogenicity and flow in the renal vasculature were within normal limits, with a resistive index of 0.74. US also revealed no signs of abnormal fluid collection or hydronephrosis. When evaluating the renal biopsy to differentiate causes of graft dysfunction, azotemia was rapidly resolved without specific treatment. His serum creatinine concentration was measured as 2.4 mg/dL 2 days after admission and as 1.4 mg/dL at the time of discharge. The exact cause of graft dysfunction remained unknown, but no further diagnostic evaluation was attempted owing to obvious clinical improvement. This episode of acute kidney injury may have been due to chronic venous stenosis and transient thrombosis in the right common iliac vein, although lower extremity edema was not apparent. His serum creatinine level returned to baseline (1.1┬Āmg/dL) 2 weeks after discharge, and remained around the baseline concentration until the development of iliac vein stenosis.
Discussion
Once considered a rare complication of renal transplantation, iliac vein stenosis has been consistently reported since 1972 [5] and shown to be related to MayŌĆōThurner syndrome in left-side transplant recipients [6], [7]. The risk factors for development of iliac vein stenosis include the prior insertion of femoral dialysis catheters, postoperative fluid collection [8], hematoma [3], urinoma, and lymphocele [9]. Renal transplant vein stenosis has also coincided with acute graft rejection [10], local infection [11], kinking of the renal vein [9], and external pressure/compression by the crossing iliac artery [12]. None of these postoperative complications was observed in our patient. It has been suggested that chronic trauma may lead to intimal proliferation with scarring and septal formation in MayŌĆōThurner syndrome [13]. Similarly, in renal transplant recipients, a local inflammatory reaction due to chronic extrinsic pressure may induce repetitive endothelial injury and subsequent vessel stenosis [2]. Surgical clamp injury, intimal dissection, and faulty suturing may also damage the vein directly, resulting in intimal proliferation and fibrosis [10]. Our patient may have experienced gradual fibrosis or scarring of the vessel wall caused by chronic compression from the bifurcation of the right common iliac artery. This may represent a rare variant of MayŌĆōThurner syndrome [14]. However, the abrupt narrowing and dilatation of the downstream vessel in our patient were not typical findings. Thus, the exact cause of iliac vein stenosis in this patient remains undetermined.
Iliac vein stenosis can be complicated by thrombosis of the iliofemoral and/or transplanted renal vein [3], [5]. Most patients present with distinctive clinical features, including lower limb edema ipsilateral to the transplant kidney and varying degrees of allograft dysfunction (Table 1). Occasionally, acquired renal transplant vein stenosis may present with similar clinical findings, although unilateral leg swelling may not occur [10], [12]. Although the clinical symptoms and signs are nonspecific, the co-occurrence of unexplained graft dysfunction combined with ipsilateral leg swelling in renal transplant recipients should be regarded as indicative of iliac vein stenosis. Other causes of renal allograft dysfunction, including ureteral obstruction, acute graft rejection, and BK virus nephropathy, should be excluded. The differential diagnosis should also include deep vein thrombosis of the lower extremity. Because slowly developing venous stenosis can progressively worsen renal allograft function but remain undiagnosed for several years after transplantation, as in our current case, a high index of suspicion should be maintained until diagnosing this rare disease.
Doppler US alone may be insufficient for identifying pathologies around the iliac vein. The absence of venous flow and/or reversed arterial diastolic flow is an indirect indication of acute venous complications in renal transplant recipients. Without thrombosis and acute pressure overload in the renal vasculature, however, chronic venous stenosis may only moderately impair venous flow, making it difficult to detect on US examination [12]. When US evaluation shows no reasons for allograft dysfunction, additional imaging methods, such as CT angiography, should be performed. Further imaging can reveal several obvious causes, including peritransplant fluid collections and deep vein thrombosis. Only by thorough evaluation, misdiagnosis and unnecessary empirical treatments for graft dysfunction can be avoided.
Interventional radiological management, including catheter-directed thrombolysis and angioplasty with or without endovascular stenting, has been found to improve allograft function in some patients with iliac vein stenosis [2], [6], [7]. Balloon angioplasty alone without stent implantation is of little use owing to unsatisfactory long-term results, including early restenosis and resistance to ballooning [10]. Iliac vein stenting has emerged as the primary treatment option for chronic venous insufficiency. Recently, iliofemoral venous stents that were purposefully designed for the iliofemoral venous segment were introduced, demonstrating better flexibility, larger diameters, longer lengths, and higher radial force [15]. To our knowledge, no previous studies have described the use of these newly developed stents to manage complications of renal transplantation. A successful endovascular stenting at the right common iliac vein of our patient restored venous patency and immediately relieved his lower extremity edema.
In summary, patients with a swollen leg and renal allograft dysfunction, even months or years after transplantation, should be suspected of having iliac vein stenosis, a rare, but potentially treatable, complication of renal transplantation. Percutaneous angioplasty with endovascular stenting is a safe and effective approach and may be the treatment of choice for managing iliofemoral venous stenosis.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















