| Kidney Res Clin Pract > Volume 31(1); 2012 > Article |
|
Abstract
We report an unusual case of probable CreutzfeldtŌĆōJakob disease (CJD) in hemodialysis patient. A woman 59 years of age with a past history of hypertension and end-stage renal disease presented with a stuporous state preceded by rapidly progressive cognitive dysfunction, myoclonus, and akinetic mutism. At first, the cause of the altered mental status was assumed to be uremic or hypertensive encephalopathy combined with fever. Proper managements, however, did not improve the neurologic symptoms. Diffusion-weighted magnetic resonance imaging revealed bilaterally asymmetric high signal intensity in both basal ganglia and cerebral cortices. Electroencephalography showed diffuse generalized theta-to-delta range slow wave and intermittent medium-to-high voltage complexes with a characteristic triphasic pattern on both hemispheres. Cerebrospinal fluid assay for the 14-3-3 protein was positive and diagnostic of CJD.
Keywords
CreutzfeldtŌĆōJakob disease, Dialysis, Hypertensive encephalopathyTransmissible spongiform encephalopathies (TSEs) are a group of rapidly progressive, fatal, neurodegenerative diseases that affect both humans and animals. The accumulation of abnormal prion proteins in the neurons is known to be the cause of TSEs [1].
CreutzfeldtŌĆōJakob disease (CJD) was the first TSE to be described in humans. It occurs in three forms: sporadic, familial, and infectious. Sporadic CJD accounts for Ōł╝85% of all CJD cases, with an incidence rate of 1 per million people a year worldwide [2]. It is rare and has not yet been reported in patients with end-stage renal disease (ESRD), although a case with Creutzfeldt(-)ŌĆōJakob-like syndrome due to drug intoxication has been reported [3], [4]. Here, we report a hemodialysis (HD) patient with altered mental status, of which the cause was revealed to be CJD.
A woman 59 years of age presented with rapidly progressive motor aphasia. She had been diagnosed with hypertension 10 years before and had been on HD for 6 years. Fifty-two days before presenting at our department, she was hospitalized due to left hemiparesis and mildly slurred speech. Brain magnetic resonance imaging (MRI) revealed right middle cerebellar peduncle infarction. She was discharged from the hospital 1 week after hospitalization.
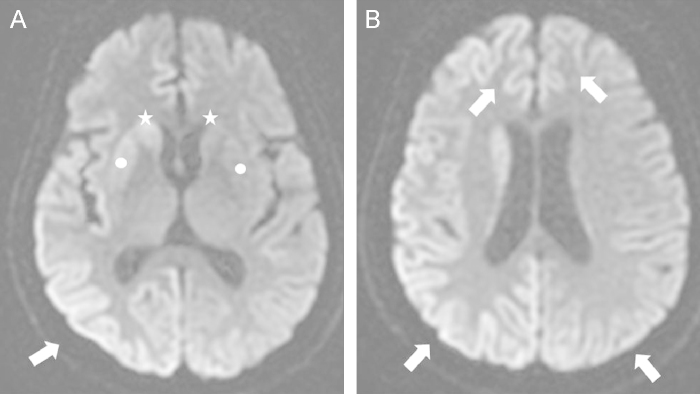
At the most recent medical visit, she presented at the emergency department with a stuporous mental status. Drug history revealed neither newly added medication nor misuse of prescribed medication. The initial vital sign showed fever (up to 38.1┬Ā┬░C) and uncontrolled hypertension (190/100┬ĀmmHg). Her mental status had deteriorated slowly but steadily over the previous 3 weeks. At this stage, neurologic examination disclosed akinetic mutism, myoclonus, and increased deep tendon reflexes (DTR) of all extremities. Preexisting slurred speech seemed to have rapidly progressed to motor aphasia, and eventually to akinetic mutism. Intermittent brief twitching of extremities had begun 2 weeks before and increased DTR and myoclonus were observed on neurologic examination. Routine laboratory studies showed elevated blood urea nitrogen (30.3ŌĆō88.7┬Āmg/dL) and creatinine (5.4ŌĆō10.6┬Āmg/dL) with hyperkalemia (6.2┬Āmmol/L). The cause of sudden mental deterioration was assumed to be hypertensive or uremic encephalopathy combined with fever. Emergency HD and empirical antibiotics was immediately started on the hospital Day 1. Emergency HD and medical treatment normalized the blood pressure, and fever disappeared after initiation of empirical antibiotics targeting urinary tract infection. Although metabolic derangements and fever were resolved, the neurologic symptoms such as the stuporous state, myoclonus and akinetic mutism did not improve. Brain MRI was performed on hospital Day 2 because of the persistent neurologic symptoms Newly developed lesions, bilaterally asymmetric high signal intensity were detected in both basal ganglia (Fig. 1A) and both cerebral cortices (Fig. 1A and B), suggesting CJD. Electroencephalography (EEG) showed diffuse generalized theta-to-delta range slow wave and intermittent medium-to-high voltage complexes with a characteristic triphasic pattern on both hemispheres (Fig. 2). Cerebrospinal fluid (CSF) examination was done on the same day, with 14-3-3 protein positivity, confirming probable CJD.
While waiting for the 14-3-3 protein assay results, which took 2 months to arrive, the patient was on maintenance HD and presented no remarkable metabolic derangements. Nevertheless, the mental status deteriorated to semicoma and the frequency of myoclonus increased.
Although a case of CreutzfeldtŌĆōJakob-like syndrome due to drug intoxication has been reported [3], [4], CJD itself had not been reported in ESRD patients so far. This patient showed progressive cognitive decline over two months, myoclonus and, akinetic mutism. EEG showed slow waves with a characteristic triphasic pattern. CSF study was positive for the 14-3-3 protein. These findings fulfill the revised World Health Organization criteria for a probable case of sporadic CJD [5].
Brain MRI was performed to exclude other possible causes of mental deterioration. Recently, hyperintensity of basal ganglia in T2- or diffusion-weighted imaging has been reported as a usual marker of CJD [6]. Because previously reported cases of CreutzfeldtŌĆōJakob-like syndrome did not show remarkable abnormal findings in brain imaging studies, the characteristic findings in brain imaging study of this patient support diagnosis of CJD.
When a HD patient presents with altered mental status and/or other abnormal neurologic signs, it is important to clarify the underlying cause, because the proper treatment for the underlying condition can ameliorate the clinical course in most cases, although it is not the case in this patient. We suggest it should be reminded CJD as a possible cause of mental deterioration in HD patients. High suspicion is needed for the diagnosis of this rare disease.
Importantly, possible alternative diagnoses were excluded in this case. Notably, thorough medical history was retrieved from her husband to exclude drug intoxication. Indeed, some cases of drug-induced encephalopathy in uremic patients have been reported, some of which even resulting in CJD-like symptoms [3], [4]. In the present case, the initial vital sign showed hypertension and fever, which could also be causes of mental deterioration. Hypertensive encephalopathy should be suspected in patients with severe hypertension and unexplained mental alteration. This medical condition requires immediate blood pressure control because of its reversibility. Brain MRI can be helpful for its diagnosis [7]. Emergency HD was performed in this patient, normalizing the blood pressure. A febrile condition can also aggravate the mental status. Therefore, the focus of fever must be sought and treated properly. Fever disappeared after treatment of urinary tract infection. Electrolyte imbalances such as hypercalcemia, hypomagnesemia, and hyper- or hyponatremia are known causes of mental alteration [8], but these were not observed in this patient.
We report a rare case of CJD in a HD patient. This diagnosis is based on the exclusion of other possible conditions that could be the cause of mental alteration in patients with ESRD. While CJD is incurable and is associated with poor prognosis, alternative diagnoses, most of which are reversible upon proper treatment, should be excluded at first. Thorough medical history, including drug history, routine laboratory studies, EEG, brain imaging studies, in particular brain MRI, and, if necessary, CSF examination, should be considered for differential diagnosis.
Acknowledgments
This research was supported by the Korea Science and Engineering Foundation through the Medical Research Center for Gene Regulation grant (2011-0030732) at Chonnam National University.
References
2. Brown P., Gibbs C.J. Jr., Rodgers-Johnson P., Asher D.M., Sulima M.P., Bacote A., Goldfarb L.G. III., Gajdusek D.C.. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 35:1994;513ŌĆō529.


3. Chuang C.L., Chen K.P., Kwan S.Y., Yang W.C., Creutzfeldt-Jakob-like E.E.G.. in a patient with end-stage renal disease. Nephrol Dial Transplant. 19:2004;252ŌĆō254.

4. Onuigbo M.A., Nye D., Iloanya P.C.. Drug-induced encephalopathy secondary to non renal dosing of common medications in two dialysis patients. Adv Perit Dial. 25:2009;89ŌĆō91.

5. Zelder M., Gibbs C. Jr, Meslin F.. WHO Manual for Strengthening the Diagnosis and Surveillance of CreutzfeldtŌĆōJakob Disease. 1998. WHO; Geneva, Switzerland.
6. Tian H.J., Zhang J.T., Lang S.Y., Wang X.Q.. MRI sequence findings in sporadic CreutzfeldtŌĆōJakob disease. J Clin Neurosci. 17:2010;1378ŌĆō1380.


Figure┬Ā1
Diffusion-weighted magnetic resonance imaging. Asymmetrically increased signal intensities in both basal ganglia (caudate nucleus and putamen: marked with stars and circles, respectively) (a) and cortical portions of both cerebral hemispheres (arrows, b), suggestive of CreutzfeldtŌĆōJakob disease.
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,874 View
- 17 Download
- Related articles
-
KaposiŌĆÖs Sarcoma in a Patient on Hemodialysis2010 July;29(4)
CrohnŌĆÖs Disease in a Patient Undergoing Hemodialysis Caused by IgA Nephropathy2009 September;28(5)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















