School urinary screening program in Japan: history, outcomes, perspectives
Article information
Abstract
In Japan, pediatric urinary screening in schools for asymptomatic hematuria and proteinuria began in 1974 and has been very successful in detecting asymptomatic kidney diseases at an early stage. While the American Academy of Pediatrics recommended discontinuing urinalysis as a public health service in 2007, urinary screening in Japan has proven extremely successful in reducing the incidence of kidney failure with replacement therapy in children and young adults, especially through the early treatment of glomerulonephritis, such as immunoglobulin A nephropathy. Furthermore, the positivity rate on urinary screening in Japan is significantly lower than in the United States where the rate of false positive results is typically very high. Japan’s seamless and efficient pediatric urinary screening may be a helpful example for other countries as well. However, the present investigation revealed several, unresolved problems with the system. For example, the methods used varied in terms of their cutoff point, additional examinations, and types of detailed testing. In Japan, various urinary screening methods are being tested to optimize the system for national use. Recently, the authors also recommended a system of detailed examinations, including beta-2 microglobulin testing and ultrasonography, to detect congenital anomalies of the kidney and urinary tract, the most common, underlying disease in kidney failure with replacement therapy, which is often overlooked until the symptoms have become grave. While school urinary screening has been ongoing for about 50 years and should be continued, improvements should also be made to it as needed.
Introduction
In Japan, a program of school urinary screening (SUS) for asymptomatic hematuria and proteinuria, begun in 1974 by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), has proved immensely successful in detecting asymptomatic kidney diseases at an early stage [1]. On the other hand, the American Academy of Pediatrics (AAP) removed urinary screening from its health supervision recommendations in 2007 [2] because of the high rate of false positive results, which led to expensive and sometimes invasive procedures with a very low, positive yield [3].
In contrast to the American experience, SUS in Japan has produced excellent results for kidney failure with replacement therapy (KFRT) and has evolved into an extremely efficient and seamless system. The present review will first discuss the history and protocol of SUS in Japan, which may be helpful in other countries, before discussing how it has contributed to reducing KFRT and which current problems need to be resolved to improve the detection of congenital anomalies of the kidney and urinary tract (CAKUT), the most frequent underlying disease in KFRT.
History of school urinary screening and comparable programs in Japan
SUS was begun in 1974 with the passage of the School Health Law in 1973. At the time, kidney disease topped the list of health-related causes of long-term absence (>50 days/year) from school, accounting for 15% of absences [4]. Early diagnosis and management of diseases in school children, including exercise programs, were considered very important for reducing absenteeism at the time. As a result, the severity of kidney disease dictated the degree of exercise restriction, with even hematuria alone considered to require exercise restriction.
In recent years, the prognosis of kidney diseases in school children has improved [5–7] thanks to progress in their treatment, leading pediatric nephrologists to recommend a relaxation of the traditional exercise restrictions. Thus, the health services involved in administering SUS transferred the focus from managing school life to a more thorough-going implementation of screening. In 2010, the Japanese Society for Pediatric Nephrology (JSPN) decided to help revise the SUS guidebook, which had previously been prepared solely by school health professionals, as a first step towards relaxing exercise management. SUS was recommended by the Japanese Society for School Health (JSSH) under the auspices of the MEXT. The Japanese guidebook was revised in 1990, 2003, 2011, and 2021 and is currently available in its fourth edition. The JSPN worked closely with the JSSH in revising the 2011 edition of the guidebook, which has been distributed to schools and municipal boards of education throughout Japan.
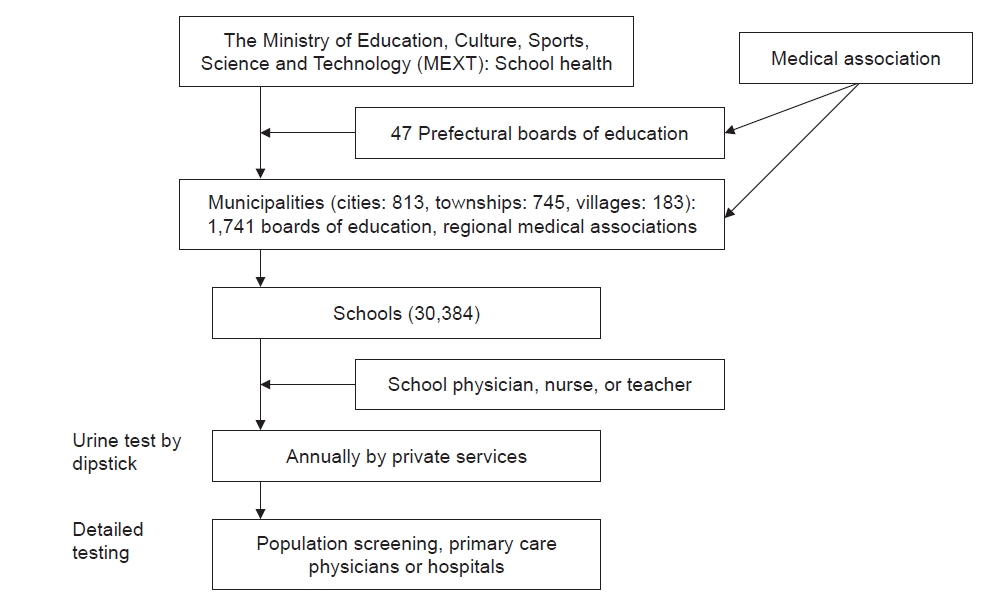
Fig. 1 shows the SUS implemented in Japan. Municipal boards of education and school health officials in regional medical associations make the final decision as to the specific methods to be employed at each school within their jurisdiction. In SUS, urinalysis is performed at each school annually by nongovernment testing services with the assistance of school physicians, nurses, and teachers. The JSSH guidebook recommends adopting certain detailed tests for use in SUS, but each municipality is able to choose its own methods.
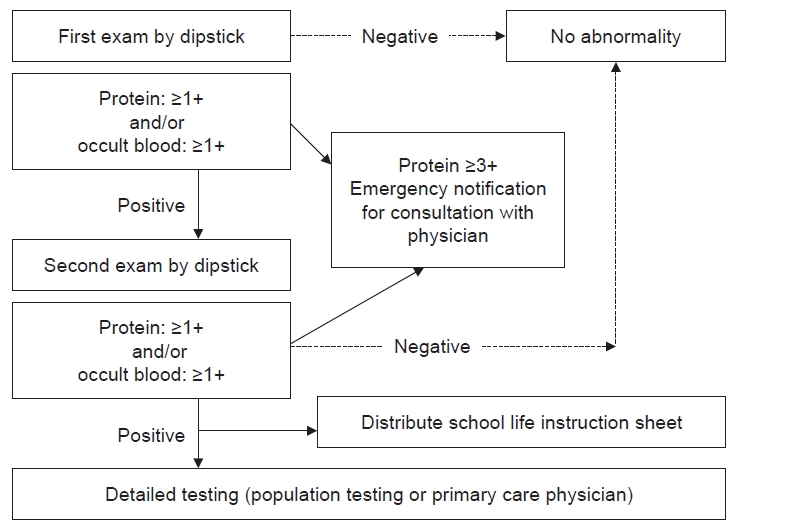
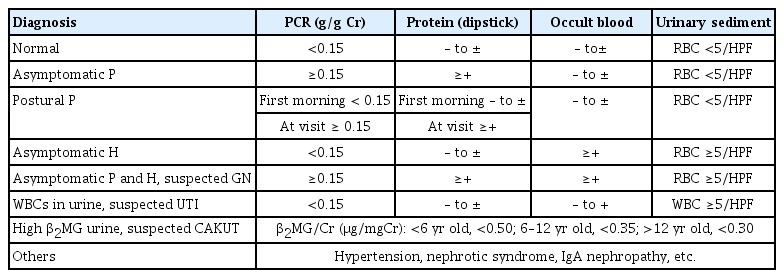
Fig. 2 shows the specific methods recommended by the JSSH guidebook for use in schools. The first-morning urine is to be tested by dipstick twice, and a cutoff of (+) is to be used for both proteinuria and occult blood (OB). An emergency notification is issued to the examinee’s parents via telephone call or post if the proteinuria value exceeds (3+). If a urine test returns positive, the student receives printed recommendations in the form of an instruction sheet for further, detailed testing. This testing is done by population mass screening or the primary physician (school physician, family physician, or health center) and includes history-taking, urinalysis, a physical examination, measurements of blood pressure, urinary protein-to-creatinine ratio (PCR), and beta-2 microglobulin-to-creatinine ratio (BMCR), and blood tests for albumin, creatinine, complement 3, etc. (Fig. 3).

Content of detailed testing (population examination or primary care physician).
CAKUT, congenital anomaly of the kidney and urinary tract; C3, complement 3; GN, glomerulonephritis; IgAN, immunoglobulin A nephropathy; NS, nephrotic syndrome; UTI, urinary tract infection; β2MG, beta-2 microglobulin; WBC, white blood cell.
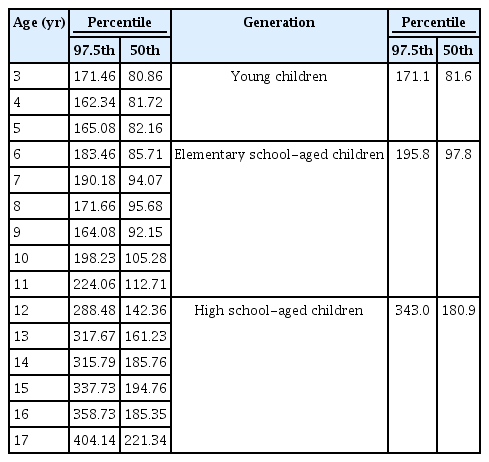
After the detailed testing is done, each physician or an evaluation committee issues a provisional diagnosis (Table 1, Fig. 3). The 2011 version includes postural proteinuria, which is generally considered to have a good prognosis based on a comparison of the first-morning urine with a sample obtained at the time of the visit. However, despite first-morning urine samples being compared twice, detailed testing still detected postural proteinuria (Table 2). Although PCR is recommended for detailed testing for proteinuria, the present review recommends the dipstick test as an alternative if PCR is unavailable. Nonetheless, a PCR value of 0.15 g/gCr or higher should be used instead of positive findings on a dipstick test whenever possible. The 2012 chronic kidney disease (CKD) guidelines published by KDIGO (Kidney Disease: Improving Global Outcomes) [8] and The Nelson textbook of pediatrics [9] suggest that the PCR reference value should be <0.2 g/gCr for the first-morning urine. However, in a previous study of Japanese subjects, the PCR value for healthy children was 0.12 g/gCr, which is the 97.5th percentile value [10]. The Japanese CKD guidelines for adults give 0.15 g/gCr as the cutoff [11], which was adopted in this study to avoid confusion.
The instruction sheet is also important as means of compiling epidemiological data because it includes the provisional diagnosis. Every school nurse using the instruction sheet is required to write down the relevant data for submission to the local board of education. The instruction sheet includes any restrictions on exercise. The school nurse plans the student’s exercise regimen based on the results. For this reason, all children who have received detailed testing are required to submit the results to their school. Although exercise restrictions have been relaxed as mentioned above, and aerobic exercise is recommended even for patients with severe proteinuria and severe kidney dysfunction, physicians may restrict strenuous activities, such as long-distance running, if they deem it necessary to do so.
Criteria for a referral to pediatric nephrologists were included in the revised 2011 edition for use in remote areas to minimize the need for consultations with specialists for only hematuria or mild urinary abnormalities, which can be dealt with by primary care physicians (Table 3). PCR findings and the proteinuria value obtained by dipstick testing were adopted as the major criteria for referring a patient to a pediatric nephrologist. In addition, if a student has gross hematuria, hypoalbuminemia, decreased serum complement, hypertension, or decreased kidney function, the primary care physician is to refer the student to a designated facility immediately. These facilities are designated by JSPN to perform kidney biopsies and diagnose and treat glomerulonephritis (GN) and other CKDs and have a pediatric nephrologist on staff.
Significance and effect of school urinary screening
Epidemiological data should be the starting point for assessing the need for screening. Recently, the number of patients with KFRT as well as KFRT caused by chronic GN (CGN) has fallen significantly. Before 1980, about 50% of cases of a primary disease associated with KFRT were of CGN, but this figure fell to 13.3% in 1998 and 5.9% in 2006 to 2011 in patients younger than 20 years. However, the frequency of CAKUT increased to about 27.6% in 1998 and 39.8% in 2006 to 2011 [5–7]. Ishikura et al. [12] surveyed primary diseases in patients younger than 15 years with stage 3 to 5 CKD. Only 1.8% of CGN cases progressed to CKD in Japan while 62% of CAKUT cases progressed to CKD, making it the main cause of the latter condition [12]. In their report on the incidence of CGN leading to KFRT in both children and adults, Yamagata et al. [13] found that only patients younger than 45 years who received SUS had a lower incidence of CKD between 1983 and 1999 (Fig. 4). However, no decrease was observed in the same period in the United States [13]. In their global report on the incidence of KFRT, Harambat et al. [14] found that in Japan the incidence of KFRT was significantly lower than elsewhere. The KFRT incidence in Japanese children aged <20 years was four per million age-related population (pmarp), which is much lower than in other, high-income countries. Eleven Western European countries and Australia on the one hand and the United States on the other hand had a pmarp of 9.5 and 15.5, respectively. The incidence of KFRT caused by GN, excluding focal segmental glomerulosclerosis, was 13.3% in Japan in 1998 and 19.6% in the United States in 1993 to 1997 [7]. The incidence fell to 5.9% in Japan according to a 2006 to 2011 survey [5] and to 10.1% in the United States in 2022. These data demonstrated that SUS was clearly successful in reducing KFRT caused by CGN in children and young adults.
The AAP removed screening urinalysis from its 2007 health supervision guidelines [15] on the grounds that many false positive results occurring in routine urinalysis led to increases in testing costs and invasive procedures with a very low positive yield [3]. Dodge et al. [16] reported that the positive rate for urinary protein was very high in the United States at 11.7% and that transient or orthostatic proteinuria accounted for a considerable number of these cases. Proteinuria (>10 mg/dL) was found in 1,440 children (11.7% of all children) in a single specimen whereas only 736 of 1,440 children (51.1%) were positive on testing of a second or third specimen collected within 2 to 7 days of the first test. Furthermore, only 18% to 27% of the 1,440 children (2.1% to 3.2%) were positive on testing of all three specimens [16]. They also stated that 210 of 340 children (61.8%) had orthostatic proteinuria. Hogg [2] reported the absence of a global consensus as to whether screening for CKD should be done in children and adolescents. However, in Japan, Korea, and Taiwan, a value of 0.4% based on two tests of early morning urine qualifies as positivity for proteinuria. These data clearly demonstrated a lower positivity rate than the figures reported by Dodge et al. [16], as mentioned above [1,17,18]. Although mass screening programs are now well established in Japan, Taiwan, and Korea, there appears to be a movement away from mass screening for CKD in children and adolescents in North America and Europe because of issues related to cost [2].
A 2013 nationwide Japanese survey [19] demonstrated that the positivity rate for protein on a dipstick test using the first urine was 0.4%, 1.5%, and 1.7% for elementary school-aged (E), junior high school-aged (J), and senior high school-aged (S) children, respectively. The positivity rate for protein on a second urine test, however, decreased to 0.1%, 0.2%, and 0.2% in the respective group. The positivity rate for OB on a secondary urine test also decreased from 0.6%–1.9% to 0.2% in all three groups. The positivity rate on a secondary urine test for both protein and OB was only 0.07, 0.08, and 0.10% in E, J, and H, respectively. These findings, which differed significantly from the United States data, indicated that only 0.3% to 0.5% of students needed further testing.
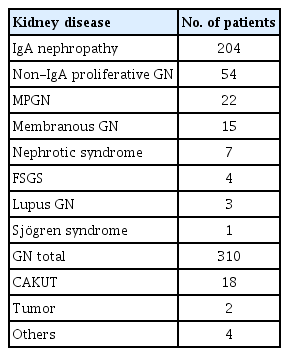
In a study conducted in Chiba City [20], the positivity rate in all students (75,000 to 129,000 per year) was 0.35% to 0.68% on urinary screening and 0.22% to 0.43% on detailed testing per year. In total, 9,544 students from 1975 to 2011 had an abnormal result after detailed testing. Of these students, 334 (3.5%) were found to have a kidney disease, and 310 (3.2%) had CGN, including 204 (65.8%) with immunoglobulin A (IgA) nephropathy (Table 4). The kidney disease detection rate was 0.6/10,000 students. Murakami et al. [1] reported about 450 students with a positive result on detailed testing; 30 of 49 of students (61.2%) who were positive for proteinuria and hematuria had GN whereas only 4.7% with hematuria alone had GN, indicating that 60% to 70% of GN cases in children were caused by IgA nephropathy. Similar results were described in Korea in students who were positive for proteinuria and hematuria [17].
Kamei et al. [21] reported on long-term survival in patients with IgA nephropathy (Fig. 5). The long-term prognosis was significantly better with combination therapy, including steroids and immunosuppressive drugs than with anticoagulation therapy for the first 2 years, suggesting that early detection and treatment are important. Of these patients, 82% were children with an average age of 12 years who were positive for protein and hematuria detected incidentally, most likely via SUS [22]. The use of combination therapy in Japan, begun in 1990, reduced the incidence of GN with KFRT and may be responsible for the dramatic drop in KFRT caused by GN in children and young adults, as previously mentioned.
A simplified, cost-benefit study found that the cost of urine dipstick testing was 300 Japanese yen (JPY) per person per examination center (personal communication to one examination center). With approximately 15 million students in Japan, the total cost of dipstick testing would run to 4.5 billion yen. The effectiveness of this method of testing was assessed on the basis of the expected reduction in the KFRT incidence. Hemodialysis costs approximately 5.5 million JPY per patient per year in Japan. If the need for dialysis could be eliminated in 82 patients over 10 years, the total cost would fall to 4.5 billion JPY. The duration of dialysis is assumed to be 10 years, but if the patient is younger than 40 years, the duration may be longer, given that the average life expectancy of dialysis patients is greater than 20 years. The incidence of IgA nephropathy in Japanese children is 4.5 to 9.9 per 100,000, with approximately 700 to 1,500 cases being diagnosed yearly [23]. KFRT is prevented in more than 10% of patients, or 70 to 150 patients with IgA nephropathy, through early diagnosis, resulting in less outlay than the cost of urinalysis. End-stage kidney disease also involves social welfare costs, such as disability payments while the costs associated with CKD are not known. These data indicate that SUS is relatively cost-effective.
Current issues in school urinary screening in Japan
In 2013, the boards of education in all municipalities and public schools throughout Japan were surveyed concerning their views on SUS and screening results. Data were obtained from 1,330 of 1,741 of municipalities (76.4%), 16,904 of 20,677 of elementary schools (81.3%), 7,885 of 9,707 of junior high schools (81.2%), and 2,959 of 3,481 of high schools (85.0%). The results revealed problems with the SUS that varied considerably by municipality [19,24].
First, each school had a different cutoff value, (±) and (+), for the dipstick test for protein and OB. Despite recommendations to use (+), the frequency of (+) vs. (±) was expressed as 40.4% vs. 56.9% for OB and 38.9% vs. 58.2% for protein. Data on provisional diagnoses were obtained from all 27,748 schools using the previously mentioned instruction sheet. Asymptomatic proteinuria and postural proteinuria, which were assessed using different cutoff levels, yielded a positivity rate of (±) twice that of (+) whereas the rate for asymptomatic protein and hematuria was identical (Table 2) [15]. These data suggested that for GN detection, (+) is better than (±) as a cutoff because it reduces the false positive rate.
The next problem was that the method of the second urine test for students with an abnormal result on dipstick testing of the first urine sample varied by school. Thirteen percent of all 27,748 schools did not perform a second urine test, increasing the rate of positive results for detailed testing. The urinary white blood cell (WBC) count via dipstick test for the second urine test was performed by 27% of all the schools, possibly contributing to the high, false positivity rate. In addition, 32% of all the schools tested for urine sediment, which should be done at the detailed testing stage because of the high costs involved. As already mentioned, only testing for protein and OB by dipstick is recommended for secondary urinalysis. However, this is problematic because further testing may increase the number of cases requiring more detailed testing as well as the cost of the second urine test.
The frequency of use of the instruction sheet also varied among schools. The instruction sheet is used not only to determine the need for exercise restrictions in each student but also to provide a provisional diagnosis for each student and to inform the board of education in each municipality about the number of abnormalities found. On average, 53% of elementary schools and 60% of junior high schools used the instruction sheet. These data indicated that about half of all municipalities were unaware of the data on students with an abnormality. Moreover, the schools were unaware of whether the students had undergone detailed testing. When each municipal educational board was asked for data on the number of students with abnormal findings and the diagnosis they received after screening, only 38% of elementary schools had information about kidney diseases in their students, compared to the figure of 49% for heart disease and 55% for allergic diseases. A similar trend was seen in junior high schools.
Detailed testing was also conducted by the municipalities on various SUS systems. For students with abnormal urinalysis findings, detailed testing was recommended via mass screening or at a designated clinic or hospital. However, only 19% of the schools surveyed conducted mass screening, and only 13% were able to provide a referral to a designated clinic or hospital for detailed testing, with 66% recommending that families find a facility for detailed testing on their own. It is unlikely that all primary care physicians are aware of the necessary tests and criteria for referral to a pediatric nephrologist. Also, not all students with abnormal SUS findings will receive detailed testing. Even in Shiga Prefecture, which has a relatively well-developed system, 5.9% and 23.6% of elementary and junior high school students, respectively, who were positive for proteinuria, did not receive detailed testing [25]. It is extremely important that children with abnormal test findings receive further, detailed testing for CKD.
Since 2011, the JSPN contributed to preparing certain sections of the JSSH guidebook but in 2021 became fully involved in all its aspects. The JSPN discussed revamping the SUS system; at present, each municipality performs its own version of SUS, and the pertinent issues were unable to be discussed with all 1,741 municipalities. Any newly developed system would ideally be adopted nationally. To this end, the JSPN is currently recommending the formation of a kidney disease committee in each prefecture (Fig. 1), with a JSPN-appointed nephrologist to work with the municipalities in each prefecture. The JSPN sent a written request and list of candidate nephrologists to each prefectural government and prefectural medical association as a first step towards forming this committee and also requested that each prefecture perform an epidemiological survey.
Because the 2011 guidebook on SUS lacked some important details, many municipalities and prefectures made their own version. JSPN decided to publish the 2015 Manual on Urinary Screening for use by family physicians, school nurses, and the relevant personnel on municipal educational boards. The specifics of the SUS methodology were illustrated in a flow chart, and 41 possible questions or issues were addressed in a question-and-answer section [26]. About 6,000 copies have already been sold. The latest edition was prepared in April 2022 [27] (Supplementary Fig. 1, available online). JSPN has also distributed a flow chart of the SUS system (Supplementary Fig. 1, available online) to each prefectural government and medical association.
Discovery of the congenital anomalies of the kidney and urinary tract and the 2022 guidebook
The current prevalence of CAKUT in Japan is 62% of CKD patients and 40% of KFRT patients as mentioned previously, but many patients with CAKUT still elude detection via SUS. The new edition of the guidebook released in 2021 contains information aimed at closing this gap in the detection rate by examining the reasons for CAKUT detection.
In patients with stage 3 to 5 CKD, only 10 of 278 of cases (3.6%) were diagnosed via urinary screening at age 3 years, and 28 of 278 (10.0%) were diagnosed on the basis of SUS results. Only 88 patients (31.7%) were found to have CKD by prenatal or perinatal ultrasound examination [12]. Wühl et al. [28] reported the rate of KFRT in patients with CAKUT with a median age of 35.1 years. Therefore, CAKUT with milder CKD must be diagnosed earlier before it progresses.
Fig. 6 shows the sensitivity of various urinary markers in patients with CAKUT [29]. In patients with CAKUT, 82% of stage 3 CKD cases and 50% of stage 2 CKD cases had a positive BMCR. When the cutoff level of protein (±) on a dipstick test was used, the positivity rate was 44% for stage 3 CKD and 30% for stage 2 CKD on a dipstick test. When the cutoff level of protein (+) was used, the positivity rate decreased to 24% for stage 3 CKD and 11% for stage 2 CKD. The urine of patients with CAKUT was diluted, with urinary creatinine of <100 mg seen in 92% of the patients. Therefore, false negative results were likely to occur when only dipstick testing was used, and BMCR was the best urine marker for detecting CAKUT. The present study found that BMCR was more useful than the dipstick test for urinary protein in detecting CAKUT with kidney dysfunction. Of course, CAKUT without kidney dysfunction can also be detected by screening [30,31], but the incidence of abnormal values in patients with CAKUT without kidney dysfunction was not examined. BMCR is also useful for detecting Dent’s disease and other tubular diseases [8].
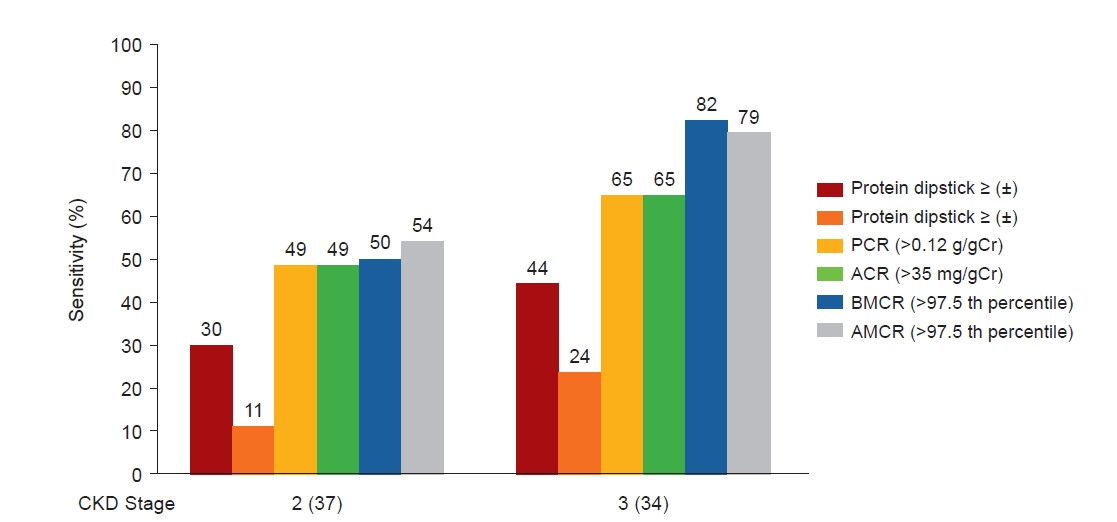
Urinary markers in patients with CAKUT. CKD stage 2–4 in 77 patients with CAKUT in Japan.
Created based on the data from the article by Hamada et al. (Pediatr Nephrol 2023;38:479–487) [29].
CAKUT, congenital anomaly of the kidney and urinary tract; CKD, chronic kidney disease; PCR, protein-to-creatinine ratio; BMCR: beta-2 microglobulin-to-creatinine ratio; AMCR, alpha-1 microglobulin-to-creatinine ratio; ACR, albumin-to-creatinine ratio; Cr, creatinine.
An analysis of the first-morning urine, randomly collected from 1,712 pupils aged ≥3 years to <18 years at school and kindergarten urinary screenings, found that the median urinary creatinine level was about 100 mg/dL for elementary school pupils but 180 mg/dL for high school students (Table 5). Because a PCR value for creatine of >0.15 g/gCr is abnormal for both age groups children, the analysis revealed that the positivity rate for high school-aged students as assessed by (+) on a dipstick test was higher than for the elementary school pupils [10].
The relationship between creatinine concentration and dipstick results bears closer scrutiny (Table 6). If (±) on the dipstick test was used and the creatinine level was found to be 100 mg, the PCR value should be within the range of 0.15 to 0.3. However, if the creatinine concentration were 50 mg, a false negative result might occur. The mean creatinine level in older children is about 200 mg. Use of dipstick (±) would produce a false positive result despite normal PCR findings. These data suggest that the cutoff of (±) on a dipstick test in children younger than 7 years old is most appropriate. However, the problem of detecting CAKUT using only a dipstick test remains.
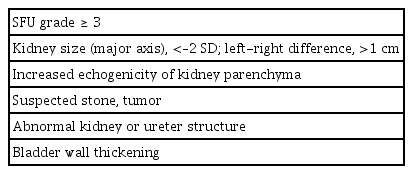
The 2022 guidebook reflects changes made to the SUS system. Among these changes is the adoption of ultrasonographic criteria for CAKUT detection. WBCs of >50/high-power field (HPF), red blood cells of >50/HPF in urinary sediment, and the reference BMCR value (0.5 μg/mgCr for 3 to 5-year-olds, 0.35 μg/mgCr for 6 to 12-year-olds, and 0.30 μg/mgCr for 12 to 18-year-olds) were also adopted as diagnostic criteria. Furthermore, criteria for referral to a specialist after ultrasonography, such as the Society for Fetal Urology grade, kidney size, presence of stones, tumors, etc. (Table 7), for detailed testing were also listed.
Conclusion
Japan’s SUS program has been in existence for almost 50 years and has achieved exemplary results. On the other hand, the AAP has removed urinary screening from health care despite its proven usefulness and cost-effectiveness in reducing KFRT. However, despite the merits of urinary screening, the present review found various problems with the system. The SUS methods used in the municipalities of Japan varied, and the low detection rate of CAKUT was also a problem. The JSPN has developed various strategies to address these issues in the hope that they will contribute to improving the efficiency and effectiveness of the nationwide system.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.23.127).
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Data sharing statement
The data used in this study for analysis are available upon reasonable request to the corresponding author.
Authors’ contributions
Conceptualization, Data curation, Formal analysis, Investigation, Methodology: All authors
Project administration, Resources: MH
Supervision: TY, YG
Writing–original draft: MH
Writing–review & editing: All authors
All authors read and approved the final manuscript.
Acknowledgements
We deeply appreciate the opportunity to present this review at KSN2021 (the virtual meeting of the Korean Society of Nephrology) held in September 2021. We thank the Board of Directors of the Japanese Society for Pediatric Nephrology and the members of the committee for CKD Task Force for their cooperation and James Robert Valera for his assistance with editing this manuscript.