| Kidney Res Clin Pract > Volume 43(1); 2024 > Article |
|
Abstract
Background
Methods
Results
Notes
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: YKK, HCP, YKL
Data curation: YKK, SJY, SY, JK, AC
Formal analysis: EK, JK, AC
Funding acquision: HCP
Investigation, Resources: SJY, SY
Methodology, Validation: EK, DHK, AC
Project administration, Supervision: HCP, YKL
Software: JK
Visualization: YKK
Writing–original draft: YKK
Writing–review & editing: all authors
All authors read and approved the final manuscript.
Acknowledgments
Figure 1.
Flow chart of the study population.

Figure 2.
Mortality of study patients.
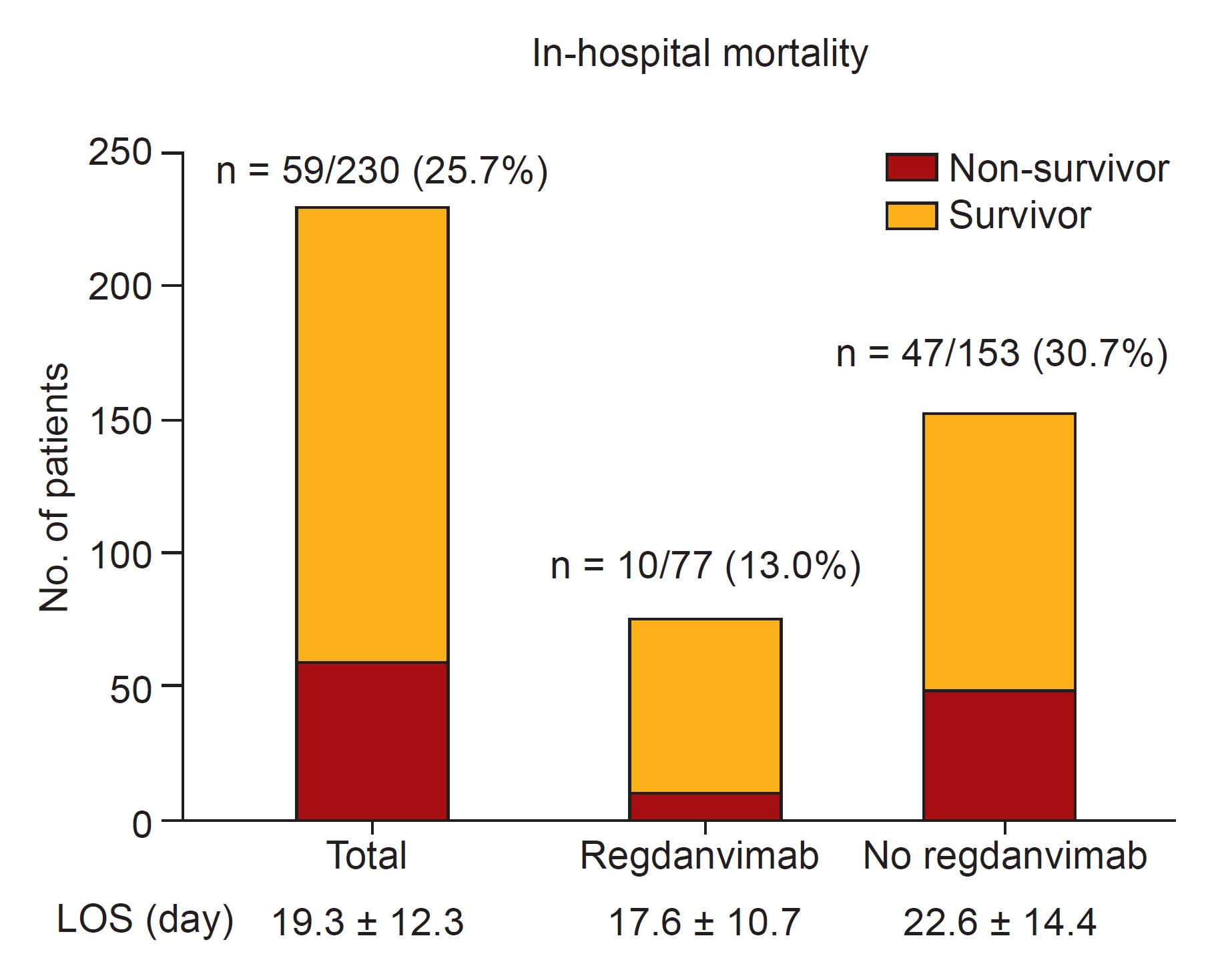
Figure 3.
Kaplan-Meier curve for in-hospital mortality according to administration of regdanvimab in total study patients (n = 230).
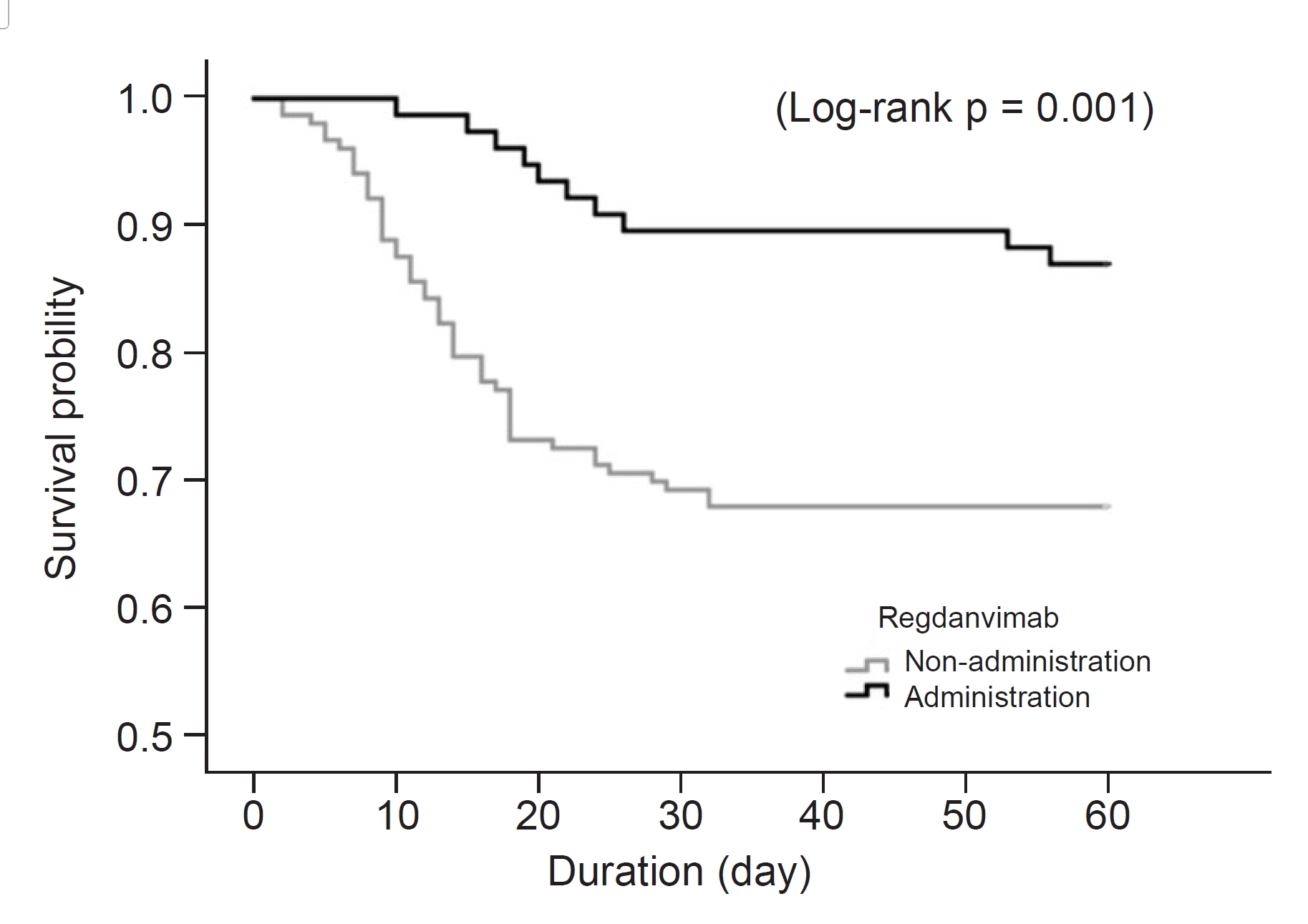
Figure 4.
Kaplan-Meier curve for in-hospital mortality according to the administration of regdanvimab in patients with SPO2 >95% (n = 206).
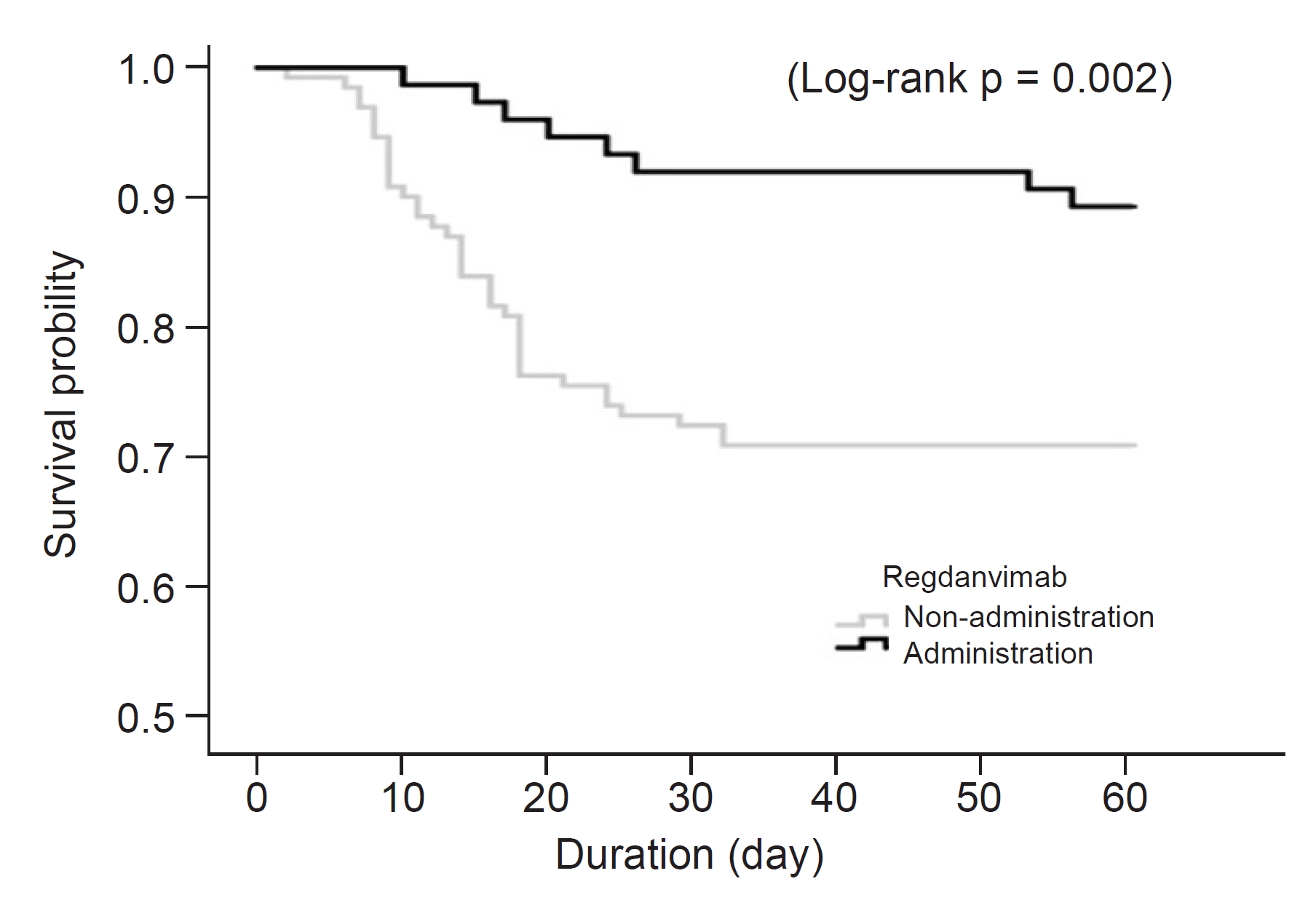
Table 1.
Data are expressed as number only, mean ± standard deviation, or number (%).
BMI, body mass index; BUN, blood urea nitrogen; BNP, brain natriuretic peptide; CAOD, coronary artery obstructive disease; CHF, congestive heart failure; CVA, cerebrovascular accident; DM, diabetes mellitus; PKD, polycystic kidney disease; RASB, renin-angiotensin system blockade; SPO2, saturation of partial pressure oxygen; WBC, white blood cell.
Table 2.
Table 3.
BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CAOD, coronary artery obstructive disease; CHF, congestive heart failure; CI, confidence interval; CRRT, continuous renal replacement therapy; CVA, cerebrovascular accident; DM, diabetes mellitus; HR, hazard ratio; ICU, intensive care unit; RASB, renin-angiotensin system blockade; SPO2, saturation of partial pressure oxygen; WBC, white blood cell.
Table 4.
BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CAOD, coronary artery obstructive disease; CHF, congestive heart failure; CI, confidence interval; CRRT, continuous renal replacement therapy; CVA, cerebrovascular accident; HR, hazard ratio; ICU, intensive care unit; RASB, renin-angiotensin system blockade; SPO2, saturation of partial pressure oxygen; WBC, white blood cell.
References
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 796 View
- 94 Download
- ORCID iDs
-
Youn Kyung Kee

https://orcid.org/0000-0002-0555-9909Hayne Cho Park

https://orcid.org/0000-0002-1128-3750Su Jin Yoon

https://orcid.org/0009-0008-3676-1799Sungbong Yu

https://orcid.org/0000-0002-1989-6121Eunsil Ko

https://orcid.org/0000-0001-5959-5545AJin Cho

https://orcid.org/0000-0001-7097-7026Do Hyoung Kim

https://orcid.org/0000-0002-8664-8830Jinseog Kim

https://orcid.org/0000-0003-3172-3212Young-Ki Lee

https://orcid.org/0000-0003-3464-6144 - Related articles
-
Risk factors for in-hospital mortality in patients starting hemodialysis2015 September;34(3)
A case of Creutzfeldt–Jakob disease in a patient on hemodialysis2012 March;31(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















