| Kidney Res Clin Pract > Epub ahead of print |
Abstract
Background
Methods
Results
Supplementary Materials
Notes
Conflicts of interest
Jong Hyun Jhee is the Deputy Editor of Kidney Research and Clinical Practice and was not involved in the review process of this article. All authors have no other conflicts of interest to declare.
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization, Methodology: DO, JHJ
Data curation: SL, EY
Formal analysis: SL, EY, DO, JHJ
Investigation: DO
Project administration: JHJ
Supervision: HYC, HCP, JHJ
Writing–original draft: DO
Writing–review & editing: All authors
All authors read and approved the final manuscript.
Figure 1.
Study subjects.

Figure 2.
The Kaplan-Meier curves of composite kidney outcome.

Figure 3.
Restricted cubic spline curve for HRs of composite outcome according to the indices in multivariable Cox regression model.
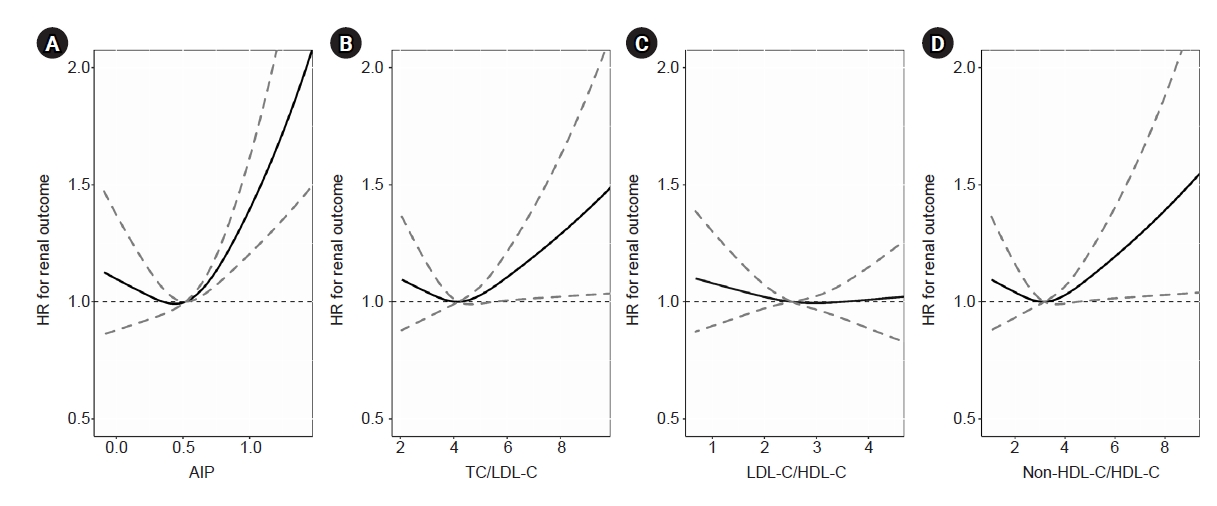
Figure 4.
Subgroup analysis for the effects of atherogenic index of plasma on the risk of composite kidney outcome.

Table 1.
Data are expressed as number only, mean ± standard deviation, or number (%).
CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration equation; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; UACR, urinary albumin to creatinine ratio; UPCR, urinary protein to creatinine ratio.
Table 2.
Model 1: unadjusted model; model 2: adjusted for age and sex; model 3: adjusted for age, sex, smoking, and alcohol status, body mass index, systolic blood pressure, hemoglobin, estimated glomerular filtration rate (Chronic Kidney Disease-Epidemiology Collaboration equation), use of lipid-lowering agents, past histories of hypertension, diabetes mellitus, coronary artery disease, cerebral infarction, dyslipidemia, and fatty liver.
AIP, atherogenic index of plasma; CI, confidence interval; HR, hazard ratio; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol.
Table 3.
Model 1: traditional risk factors only; model 2: traditional risk factors + eGFR; model 3: traditional risk factors + eGFR + atherogenic index of plasma. Traditional risk factors: age, sex, status of smoking and alcohol, body mass index, systolic blood pressure, hemoglobin, eGFR, medication history of lipid-lowering agents, and past histories of hypertension, diabetes mellitus, coronary artery disease, cerebral infarction, dyslipidemia, and fatty liver.
CI, confidence interval; eGFR, estimated glomerular filtration rate; IDI, integrated discrimination improvement; NRI, net reclassification index.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 790 View
- 28 Download
- ORCID iDs
-
Donghwan Oh

https://orcid.org/0000-0001-7570-0288Seoyoung Lee

https://orcid.org/0000-0001-9467-8856Eunji Yang

https://orcid.org/0000-0001-7039-5093Hoon Young Choi

https://orcid.org/0000-0002-4245-0339Hyeong Cheon Park

https://orcid.org/0000-0002-1550-0812Jong Hyun Jhee

https://orcid.org/0000-0002-1255-1323 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















