Introduction
The increase in patients with end-stage renal disease (ESRD) has primarily occurred in the elderly population. In 1985, 36% of the ESRD population was over 65 years of age. By 2009, the proportion had increased up to 48% according to United States Renal Disease System data [
1]. In Korea, the number of people waiting for kidney transplantation (KT) has increased in the elderly population, and the number of people who have undergone KT has also increased. The proportion of patients over 60 years of age who underwent KT in 2006 was only 4.4%. However, it had increased to 21.9% in 2019 [
2]. Elderly recipients are associated with a higher comorbidity. However, advanced age itself is not a contraindication of KT [
3]. Previous studies have shown KT significantly reduces mortality compared to dialysis in a waiting-list elderly population with ESRD [
3,
4]. The elderly recipients have improved their quality of life after KT [
5].
Aging affects all aspects of adaptive and innate immunities [
6]. Immunosenescence gradually deteriorates the function of immune system, making the elderly susceptible to infection and malignancy, and eventually contributes to morbidity and mortality [
7–
9]. Hemmersbach-Miller et al. [
10] reported that elderly recipients have a higher incidence of urinary tract infection (UTI) and more cytomegalovirus (CMV) reactivation, which usually occurs within 1 year after KT. Furthermore, Lim et al. [
11] reported a significantly higher mortality rate from infection in elderly recipients compared to younger recipients. Modifications of T-cell function are the result of immunologic changes with aging and are a factor that reduces acute rejection in elderly recipients [
8]. As such, the consequences of immunosenescence have a wide range of effects on organ transplants, and age-adaptive immunosuppression may be required in elderly recipients [
12]. In previous studies, the comparison of induction agents has mainly focused on elderly patients. There have been reports suggesting that patient survival is worse when rabbit anti-thymocyte globulin (r-ATG) is used at a dose higher than 6 mg/kg [
13]. Additionally, compared to a dose of 2.5 mg/kg, similar rejection rates were observed, but there was a higher incidence of infections [
14]. Moreover, previous studies reported that using r-ATG at a dose higher than 4.5 mg/kg leads to a higher risk of infection compared to basiliximab (BSX) [
15,
16].
In this study, we evaluated the clinical outcomes, including rejection and infection rate when using BSX and r-ATG as induction agents, in elderly and young recipients with the same maintenance immunosuppressive agents.
Methods
This retrospective study was approved by the Institutional Review Board of Samsung Medical Center (No. 2020-12-045), and the need for informed consent was waived.
Patients and data
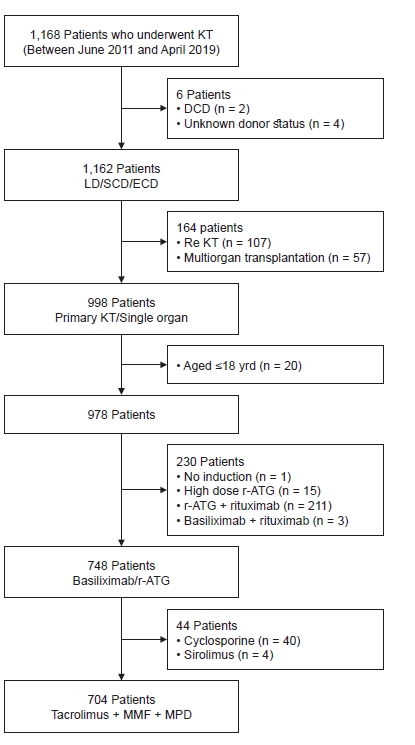
We retrospectively reviewed all patients who underwent KT at Samsung Medical Center in Seoul, Korea, between June 2011 and April 2019.
Fig. 1 shows a flow chart summarizing patient enrollment. We included 1,162 patients without any cardiac death and unknown donors. Primary KT and single organ transplantations were included. We excluded patients younger than 18 years of age and those who did not receive immunosuppressive induction therapy or a combination of rituximab and r-ATG or BSX. We also excluded patients who received high dose r-ATG (1.5 mg/kg for more than 5 days) because r-ATG dose could affect not only recipient infection but also biopsy-proven acute rejection (BPAR) [
15]. Recipients who received cyclosporin or sirolimus as initial maintenance immunosuppressive therapy were also excluded. Finally, 704 patients were enrolled in this study.
Immunosuppression
The immunosuppressive induction agents used were BSX and r-ATG. We classified patients according to their immunologic risk as follows. Low-risk patients were those without preoperative donor-specific antibody (DSA), and intermediate-risk patients were those with preoperative DSA and median fluorescence intensity (MFI) less than 2,500 in the panel reactive antibody (PRA) assay. High-risk patients were those with preoperative DSA and MFI higher than 2,500 in the PRA assay. We usually use BSX for immunologic low-risk patients in living donor (LD) KT and patients who receive standard criteria donor kidneys from donation after brain death (DBD) donors and r-ATG for immunologic intermediate and high-risk patients in LD KT and patients who receive extended criteria donor (ECD) kidneys from DBD donors. However, we gave induction agents based on the condition of each patient, including immunologic risks and physical conditions. Recipients who received 20 mg of BSX as an induction agent were injected intravenously twice on the operative day and on postoperative day 4. r-ATG (1.5 mg/kg for 3 days) was initiated on the operative day and administered daily. Intravenous methylprednisolone (MPD, 500 mg) was also administered for 2 days starting on the operative day and tapering as scheduled.
Maintenance immunosuppression was achieved with a triple immunosuppressive regimen consisting of tacrolimus, mycophenolate mofetil, and MPD.
Human leukocyte antigen single identification and postoperative donor-specific antibody evaluations
Human leukocyte antigen (HLA) mismatches were preoperatively evaluated, and the analysis included class I mismatches (HLA-A and HLA-B) and class II mismatches (HLA-DR) only. The HLA single identification was performed using a Luminex assay (Luminex Corporation). We performed postoperative DSA evaluations at 4 weeks, 6 months, and 1 year after transplantation, followed by annual evaluation using Luminex assay to check for the occurrence of de novo DSA.
Antimicrobial prophylaxis
Pneumocystis jiroveci pneumonia (PJP) prophylaxis with sulfamethoxazole-trimethoprim is performed for 6 months when r-ATG is used as an induction agent and 6 weeks after steroid pulse therapy due to acute rejection. Antifungal prophylaxis with itraconazole is performed for 2 weeks when r-ATG is used as an induction agent. CMV prophylaxis depends on CMV serostatus. In the case of CMV seropositive recipients, preemptive monitoring through a CMV antigenemia test was performed for 12 weeks and antiviral therapy (ganciclovir or valganciclovir) was initiated in patients with CMV antigenemia of more than 50/400,000 white blood cells. For CMV seronegative recipients who received kidneys from CMV seropositive donors, CMV prophylaxis was performed for 6 months. Even for CMV seropositive recipients, CMV prophylaxis was performed for 2 weeks when r-ATG was used as an induction agent.
Clinical parameters and outcomes
BPAR was defined and classified according to the Banff 2013 classification. At our center, a protocol biopsy is performed at 2 weeks and 1 year after KT from 2012 [
17]. We investigated infections such as CMV, BK virus, fungal infection, pneumonia, UTI, and bloodstream infection (BSI), and when each infection first occurred. CMV was detected by antigenemia, and BK virus was detected by plasma polymerase chain reaction. The evaluation of CMV antigenemia and BK viremia was performed monthly until 1 year after transplantation. UTI was defined as more than 100,000 colony-forming units per milliliter on urine cultures with urinary symptoms. Pneumonia was defined as positive radiologic findings and two or more symptoms of cough, sputum, rigors, dyspnea, and fever. PJP was also included in pneumonia patients. BSI was defined as one or more positive blood cultures associated with systemic signs of infection such as fever, chills, and/or hypotension. Patient death due to infection was defined as cases where infection was identified as the primary cause of death among post-transplantation patients. Among all cases, patient deaths were attributed to bacterial or viral pneumonia, PJP, and invasive aspergillosis pneumonia. Additionally, we observed other causes of patient death, including cardiovascular disease, cerebrovascular disease,
de novo cancer, as well as cases where the cause remained undetermined.
Statistical analyses
Continuous variables are presented as mean ± standard deviation and were compared using the Student t test and one-way analysis of variance or as medians with interquartile ranges, which were compared using the Kruskal-Wallis test. Categorical variables are presented as numbers and percentages and were compared by chi-square tests. Graft failure was defined as restarting dialysis or retransplantation. Graft survival was estimated using the Kaplan-Meier method and compared using the log-rank test. Logistic regression analyses were used to find risk factors for each infection. Multivariate analysis was performed using the factors from the univariate analysis that were statistically significant (p < 0.05) and clinically significant factors that were not statistically significant in the univariate analysis. All tests were two-tailed, and statistical significance was defined as p < 0.05. All statistical analyses were conducted using IBM SPSS version 25.0 (IBM Corp.).
Discussion
In this study, we observed similar occurrences of BPAR and most infections, except CMV antigenemia and UTI, within 6 months between the younger and older recipient groups when BSX was used as an induction agent. However, when r-ATG was used as an induction agent, BPAR within 6 months occurred less frequently in older recipients, while all infections within 6 months as well as deaths due to infection were more common in older recipients. In the older recipient group, BPAR within 6 months occurred less frequently, but CMV antigenemia within 6 months was more frequent when r-ATG was used as an induction agent compared to BSX. Furthermore, despite the older recipient receiving kidneys of poorer quality, there was no significant difference in death-censored graft survival compared to the younger recipient group. In multivariate analysis, recipient age equal to or over 60 years and the use of r-ATG as an induction agent were risk factors for viral infections such as CMV and BK virus within 6 months.
Worldwide, it is estimated that approximately 40% of patients with ESRD are 65 years of age or older, a proportion that is steadily increasing [
18]. Previous studies have shown that for these elderly patients, undergoing KT could improve patient survival compared to remaining on the waiting list [
19]. However, it should be noted that for elderly patients, the risk of mortality may be higher up to 1.8 years post-KT compared to remaining on dialysis [
4]. Therefore, potential candidates for KT should be carefully evaluated considering comorbidities, frailty, and physical and cognitive function [
19]. In addition, even in elderly patients undergoing KT, it is necessary to consider the modifications of induction agents due to alterations in the immune system caused by immunosenescence. Previous studies have reported an increased rate of infection and malignancy when using r-ATG as an induction agent in elderly recipients, compared to BSX [
16,
20]. In contrast, Goumard et al. [
21] observed a higher incidence of BPAR when using BSX as an induction agent in sensitized elderly recipients.
Previous studies showed that the incidence of acute rejection decreased as the age of the recipient increased [
22–
24]. This reduction in acute rejection is directly related to age-related immune dysfunction as a part of immunosenescence [
8]. The sensitization ability of the innate immune system as well as the response by the adaptive immune system are reduced with aging [
12]. In contrast, old donors are reported to show increased acute rejection rates due to increased immunogenicity [
12,
25]. Rana et al. [
24] reported that recipient age was a robust factor for acute rejection within 1 year regardless of the type of induction agent. Similarly, in our study, the incidence of BPAR within 6 months was lower in older recipients than in younger recipients. In addition, the incidence of BPAR within 6 months was less in the older group than the younger group when r-ATG was used as an induction agent. However, there was no difference in the incidence of BPAR within 6 months between the younger and older groups when BSX was used as an induction agent (
Table 2). In multivariate analysis, young age and the use of BSX as an induction agent were risk factors for BPAR within 6 months (
Supplementary Table 2, available online). In contrast, rejection-free graft survival was similar in the long-term between older and younger recipients regardless of the type of induction agent (
Fig. 2B). Dreyer and de Fijter [
18] reported that HLA class II mismatch influenced the formation of
de novo DSA, leading to an increase in BPAR. Similarly, our results showed that HLA class II mismatch was an independent risk factor for BPAR within 6 months (
Supplementary Table 2, available online).
In contrast to acute rejection, the incidence of infection increased as the age of the recipient increased [
8,
26]. In addition, the relative risk of death due to infection was considerably affected as the age of the recipient increased [
22]. Dharnidharka et al. [
27] reported that bacterial infection increases with age of the recipient, whereas viral infection decreases with age of the recipient. However, Pham et al. [
16] reported that the incidence of viral infection as well as bacterial infection was higher in patients over 65 years of age within 1 year after KT. Hemmersbach-Miller et al. [
10] reported that the incidence of UTI and CMV were significantly higher in older recipients within 1 year after KT, but the incidence of pneumonia and BSI did not differ according to age. In our study, bacterial, viral, and fungal infections were all higher in the older recipient group within 6 months after KT. However, the incidence of all infections was higher in older recipients when r-ATG was used as an induction agent, but only the incidences of CMV and UTI were higher in older recipients when BSX was used as an induction agent. In addition, patient death due to infection did not differ according to age when BSX was used as an induction agent, but it occurred more frequently in older recipients when r-ATG was used as an induction agent (
Table 2). In multivariate analysis, old age and the use of r-ATG as an induction agent were risk factors for CMV antigenemia and BK viremia within 6 months (
Table 3,
4).
Elderly patients who receive a kidney from an ECD demonstrate improved survival outcomes compared to those who remain on the waiting list in anticipation of a transplant from a higher-quality donor [
28]. Doucet et al. [
29] reported similar findings, with elderly patients more likely to receive kidneys from either ECD or elderly donors, according to data from the Australia and New Zealand Dialysis and Transplant Registry. Despite the lower quality of donors in elderly patients, there was no significant difference in death-censored graft survival compared to younger recipients. Heldal et al. [
30] found that the 5-year death-censored graft survival rate of 89% in KT recipients aged over 70 years, which was comparable to that of senior patients aged 60–69 years and control patients aged 45–54 years. Faravardeh et al. [
31] reported no significant difference in the 15-year death-censored graft survival among KT recipients aged under 50, 50–64, and 65 years and older. Similarly, in our study, older recipients were more likely to receive kidneys from donors of advanced age or ECDs, and these donors were more likely to have a history of DM, HTN, or higher terminal creatinine levels. Furthermore, our study showed no significant difference in death-censored graft survival between the younger and older recipient groups.
Clinical trials on the use of immunosuppression in the elderly are rare, so there are still no guidelines for age-adapted immunosuppression. Krenzien et al. [
26] recommended the type and dose of induction therapy according to the immunologic risk in the elderly. For sensitized elderly recipients such as the second KT, 6 mg/kg of r-ATG was recommended. In non-sensitized elderly recipients, it is recommended to reduce the r-ATG up to 3 mg/kg for marginal organ quality such as ECD or ischemic time over 24 hours, and to use BSX for good organ quality such as a living donor or short ischemic time. Masset et al. [
32] conducted a comparative study on the use of BSX and r-ATG at a dosage of 1.5 mg/kg/day for an average of 6.8 days in elderly KT recipients with low immunological risk. They found no significant difference between the two groups in the outcomes of graft and patient survival, BPAR, infection, and malignancy. However, they observed a lower incidence of posttransplant diabetes in the r-ATG group, which was attributed to the use of low-dose tacrolimus. In a study by Patel et al. [
13] that divided patients into four groups based on age (60 years) and r-ATG 6 mg/kg, the patient survival rate was significantly lower when r-ATG over 6 mg/kg was used in patients over 60 years compared to other groups. Pham et al. [
16] reported that the occurrence of CMV infection was significantly higher when r-ATG 4.5 mg/kg was used compared with when BSX was used in patients over 65 years of age. El-Hag et al. [
15] reported that elderly recipient treated with 4.5 or 6 mg/kg r-ATG experienced a higher rate of infections such as CMV, BK, and UTI than the BSX and 3 mg/kg r-ATG groups. Yang et al. [
14] reported that low-dose r-ATG (2.5 mg/kg) had a BPAR similar to that of 6 mg/kg r-ATG, but a statistically significantly lower incidence of infection. Cabrera et al. [
33] reported that when extremely elderly recipients, 75 years of age and above, received kidneys from extremely aged deceased donors, interleukin-2 receptor antagonist was used as an induction agent in 91.9% of cases. In our study, when 4.5 mg/kg r-ATG was used as an induction agent in older recipients, the incidence of BPAR was low (
Table 2), but the incidence of infection was high and more patients died from infection (
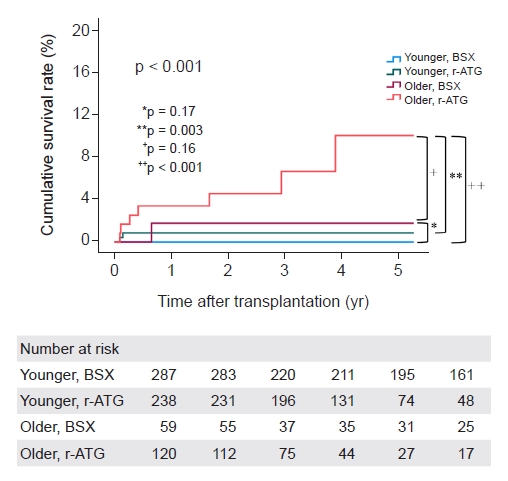
Fig. 3). Thus, we speculate that 4.5 mg/kg of r-ATG might be a bit excessive in older recipients. A prospective study is needed to determine how much the r-ATG dose should be reduced.
This study is subject to similar limitations inherent to all retrospective studies, including missing data elements, protocol deviations, and selection bias. These problems can limit the ability to draw causal conclusions. To address potential confounders, we performed a multivariate analysis adjusting the recipient’s age, sex, and other relevant covariates. However, residual confounding may still exist, especially for the time point of BPAR and outcome events. Our results cannot be generalized to other centers using different induction strategies. In addition, the incidence of BPAR is relatively high because our center performs protocol biopsy in the second week. Thus, a prospective multicenter study is needed to confirm our findings.
In conclusion, when BSX was used as an induction agent, there were similar rates of BPAR and most infections between the younger and older recipient groups. Although CMV and UTI occurred more frequently in older recipients, infection-related mortality was comparable between the two groups. However, when 4.5 mg/kg of r-ATG was used as an induction agent in older KT recipients, not only did all infections occur more frequently, but the incidence of infection and death due to infection were higher compared with younger recipients. Considering the potential excessive dosage of 4.5 mg/kg for r-ATG in older recipients compared to younger recipients it may be necessary to use less intensive induction therapy for older recipients, of which dose reduction of r-ATG is one option.













 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















