| Kidney Res Clin Pract > Volume 42(4); 2023 > Article |
|
See the article "Genetic variations in HMGCR and PCSK9 and kidney function: a Mendelian randomization study" in Volume 42 on page 460.
Genetic analysis [1,2], particularly the Mendelian randomization approach, has become one of the most important studying tools for contemporary life science, including lipidology and pharmacology. In the current issue, Park et al. [3] demonstrated that genetically predicted HMGCR inhibition is associated with a low estimated glomerular filtration rate (eGFR), while that of PCSK9 is associated with high eGFR using a Mendelian randomization study. Impaired renal function is one of the leading public health burdens. At present, a considerable number of individuals are taking lipid-lowering agents, and many of them have multiple comorbidities. Particularly, many elderly people and patients with chronic kidney disease are candidates for statin prescription [4,5]. To date, it has not been frequently agreed that statins have harmful effects on renal function. Although an excess of postoperative acute kidney injury after rosuvastatin use has been reported [6], this finding was not regarded as serious and included in the latest guidelines [7].
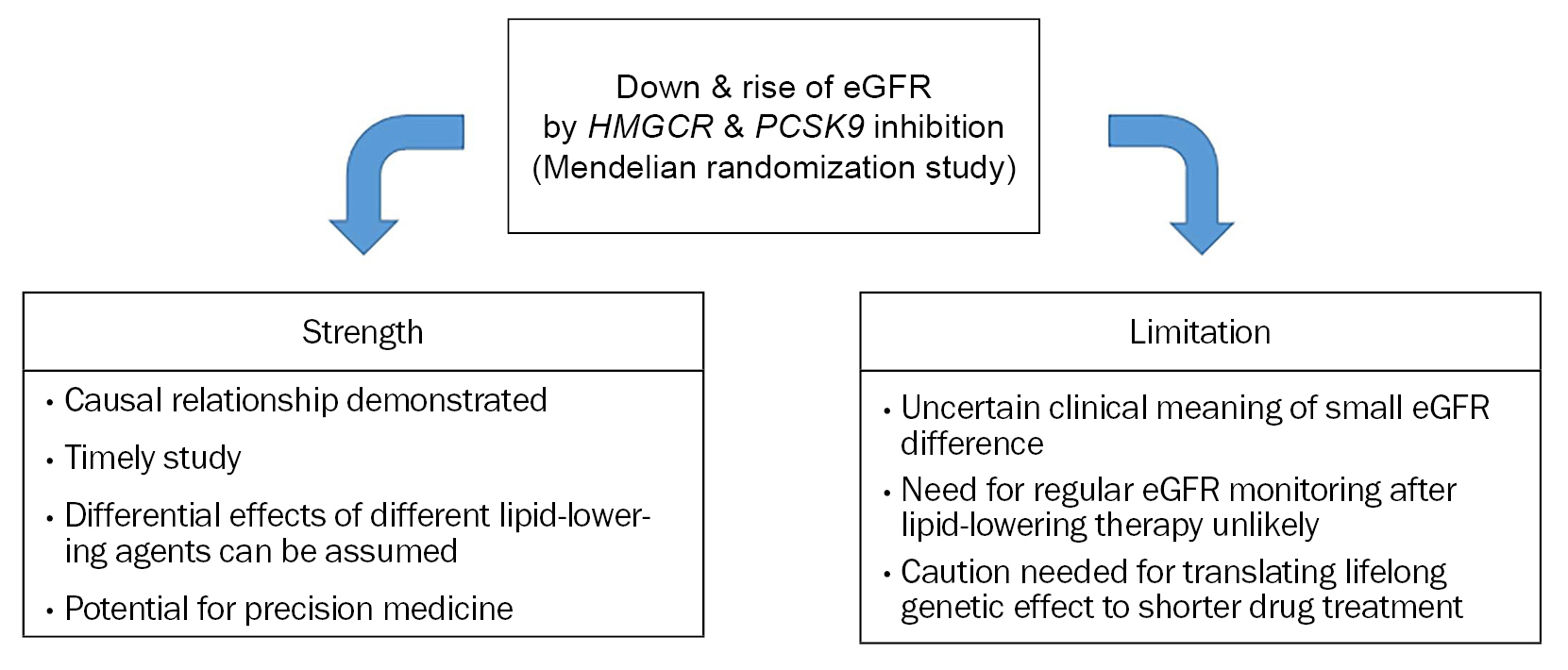
Strong points of the study by Park et al. [3] are as follows: 1) It analyzed the causal relationship between lipid-lowering and renal function using eGFR, which has not been well established yet. 2) It is a timely study of the current health environment in which both renal dysfunction and statin use are becoming more common. 3) It newly identified differential effects of inhibition of HMGCR and PCSK9 on renal function. Studies on the impact of variants of lipid-related genes on nonlipid parameters have potential importance in medicine. This can be utilized to coin polygenic scoring system that can be applied to select individuals who can experience additional benefit or harm from specific lipid-modifying drugs (so-called “precision medicine”). The size of benefit or harm has not been fully clarified by previous studies including that by Park et al. [3].
However, some issues need to be pointed out in this study. Lipid-lowering efficacy of statin is commonly presented as percentage changes. The study by Park et al. [3] used absolute differences in low-density lipoprotein cholesterol (LDL-C) rather than its percentage difference. When performing lipid-lowering pharmacotherapy, for instance with statins, the drug intensity by which a person can obtain 50 mg/dL of LDL-C reduction might be diverse. Low- to moderate-intensity statins can reduce >50 mg/dL if the baseline LDL-C is 200 mg/dL, whereas it might be difficult to decrease the level by 50 mg/dL even with high-intensity statins if the baseline level is 80 mg/dL. Thus, when we try to estimate the effect of LDL-C differences, it may cause confusion if the absolute LDL-C values are used because the baseline LDL-C range is wide in the real world.
Although the LDL-C difference of 50 mg/dL is quite large for most people, the differences in eGFR affected by variations of two genes are quite small. In this regard, clinical impact of these two genetic pathways and that from these eGFR changes needs to be interpreted cautiously. In some prior Mendelian randomization studies on lipid traits, the risk differences of coronary artery disease according to genetic variation of lipid-related genes have been greater than 40% [8,9]. Compared to these findings, clinical implication of small eGFR changes affected by lipid-related genes seems to be limited. This difference between studies could be partly from the relatively weak relationship between lipids and renal function compared to that between lipids and atherosclerotic vascular disease (Fig. 1).
It seems better to consider several points when clinicians accept the results of Park et al. [3]. The benefit of regular monitoring of renal function for statin therapy is not clear and is not currently recommended. This study used the Mendelian randomization approach that represents lifelong effect of genetic variation. However, the impact of LDL-C difference on eGFR was modest, and this impact can be smaller when the difference is made by pharmacotherapy in a certain life period rather than lifelong duration. In this regard, it needs caution when interpreting the results on HMGCR and PCSK9 inhibition and extrapolating them to the lipid-lowering drug effects.
Figure 1.
Strength and limitation of Park et al.’s study [3].
eGFR, estimated glomerular filtration rate.
References
1. Lee SH. Role of genetics in preventive cardiology: focused on dyslipidemia. Korean Circ J 2021;51:899–907.




2. Kim B, Joo Lee C, Won HH, Lee SH. Genetic variants associated with supernormal coronary arteries. J Atheroscler Thromb 2023;30:467–480.


3. Park S, Kim SG, Lee S, et al. Genetic variations in HMGCR and PCSK9 and kidney function: a Mendelian randomization study. Kidney Res Clin Pract 2023 May 22 [Epub]. DOI: 10.23876/j.krcp.22.237.

4. Razavi AC, Mehta A, Sperling LS. Statin therapy for the primary prevention of cardiovascular disease: Pros. Atherosclerosis 2022;356:41–45.


5. Yang YS, Kim HL, Kim SH, Moon MK; Committee of Clinical Practice Guideline, Korean Diabetes Association and Clinical Practice Guideline Committee. Lipid management in Korean people with type 2 diabetes mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis consensus statement. J Lipid Atheroscler 2023;12:12–22.




6. Zheng Z, Jayaram R, Jiang L, et al. Perioperative rosuvastatin in cardiac surgery. N Engl J Med 2016;374:1744–1753.


7. Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019;290:140–205.


-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,105 View
- 66 Download
- ORCID iDs
-
Sang-Hak Lee

https://orcid.org/0000-0002-4535-3745 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















