Removal of uremic toxin by dialysis, what is the issue?
Article information
As renal function deteriorates, uremic toxins accumulate in the body and are traditionally classified as small water-soluble compounds, protein-binding compounds, and middle molecules. These accumulated uremic toxins not only cause various uremic symptoms, such as nausea, vomiting, anorexia, fatigue, pruritus, mental status changes, and restless leg syndrome, but are also associated with mortality and morbidity [1]. Therefore, efficient removal of these uremic toxins through dialysis is a carefully considered therapeutic strategy by nephrologists. Removal of these uremic toxins is thought to improve uremic symptoms and clinical outcomes. Among the uremic toxins, small water-soluble compounds are effectively removed by conventional hemodialysis (HD), whereas protein-bound compounds and middle molecules are not. However, technological advances, including high-efficiency hemodiafiltration (HDF) and the development of new HD membranes, such as medium cutoff (MCO) dialyzers, have made it possible to remove molecules of up to approximately 50 kDa [2].
Recent studies have reported that high-volume HDF improves clinical outcomes, such as all-cause mortality [3]. The survival benefit of high-volume HDF is thought to be partly related to the removal of protein-bound compounds and middle molecules. Because MCO-HD is as effective as high-volume HDF in the removal of protein-binding compounds and middle molecules, MCO-HD is expected to show survival benefits similar to those of high-volume HDF [4]. However, unlike high-volume HDF, no study has reported that MCO-HD shows a survival benefit. Moreover, the results of previous studies on the effects of MCO-HD on the removal of middle molecules have been inconsistent [4,5].
In this respect, the paper titled “Comparison of the medium cutoff dialyzer and postdilution hemodiafiltration on the removal of small and middle molecule uremic toxins” published in Kidney Research and Clinical Practice by Kim et al. [6] is interesting. In this prospective non-randomized crossover study involving nine patients, Kim et al. [6] compared the small and middle molecule clearance of MCO-HD with that of postdilution HDF. There was no difference in the removal of uremic toxins under 12,000 Da between high-flux HD, MCO-HD, and postdilution HDF, which is consistent with the results of previous studies [5]. However, Kim et al. [6] reported that MCO-HD was more effective than postdilution HDF for the middle molecules. Among the middle molecules, there was no significant difference in the reduction ratio (RR) of β2-microglobulin (B2MG) (HDF vs. MCO-HD, 67.9% ± 11.7% vs. 71.6% ± 5.7%; p = 0.26), but myoglobin, kappa free light chain (FLC), and lambda FLC (HDF vs. MCO-HD, 15.8% ± 8.5% vs. 49.8% ± 6.5%; p = 0.008) were significantly higher in MCO-HD than in postdilution HDF. However, these results are inconsistent with those of previous studies [4,5]. In 2022, Hadad-Arrascue et al. [4] compared the clearance of middle molecules in postdilution HDF (n = 21) and MCO-HD (n = 22) in an open randomized clinical study. In this study, the RRs of B2MG, kappa FLC, and lambda FLC were not significantly different between the two groups. In 2022, Kim et al. [5] conducted a study with a design very similar to that of Kim et al. [6] and compared the clearance of HF-HD, postdilution HDF, and MCO-HD for urea, B2MG, indoxyl sulfate (IS), p-cresyl sulfate (pCS), kappa FLC, and lambda FLC [5]. There was no significant difference in urea clearance between dialysis modalities, as reported by Kim et al. [6]. However, the RR of B2MG was significantly higher in postdilution HDF than in MCO-HD (HDF vs. MCO-HD, 79.54% ± 4.72% vs. 75.32% ± 4.64%; p < 0.001). On the other hand, the RR of lambda FLC was significantly higher in MCO-HD than in postdilution HDF, similar to the study by Kim et al. [6] (HDF vs. MCO-HD, 43.48% ± 7.41% vs. 51.52% ± 6.08%; p < 0.001). This discrepancy in the results is likely due to differences in the study design. The study by Kim et al. [5] differed from that by Kim et al. [6] in that it was a randomized study with more patients (22 patients), used a hemodiafilter with a larger inner diameter for postdilution HDF treatment, and had a higher convection volume. In particular, the possibility that the use of a hemodiafilter for HDF (FX 800) instead of a high-flux dialyzer (FX 80) during HDF treatment affected the clearance of B2MG cannot be ruled out. Therefore, these limitations must be considered when interpreting the results of Kim et al.’s study [6].
As in this study, there are several points to consider when conducting research on the removal of uremic toxins by dialysis or interpreting the results. First, when HD is performed intermittently thrice a week, the unpredictable effect of kinetics on the removal of various uremic toxins must be taken into account [7]. Therefore, when evaluating the ability to remove uremic toxin, the predialysis concentration after a sufficiently long equilibration (≥4 weeks) might be a better measure than the RR calculated by measuring the blood concentration before and immediately after the end of dialysis [1,8], as in the study by Kim et al. [6] (Fig. 1). An equilibration time of 4 weeks allows most of the solutes to reach equilibrium while minimizing the occurrence of confounding factors caused by residual kidney function, use of antibiotics, dialytic prescription, and changes in dietary intake [1]. Second, it is necessary to determine the association of removal of uremic toxins with clinical outcomes and quality of life measures [1,9]. Uremic toxins are traditionally classified as small water-soluble compounds with low molecular mass (<500 Da), protein-bound solutes, and middle molecules (≥500 Da). This classification was developed by the European Uremic Toxin (EUTox) work group in 2003 based on their physicochemical properties that affect removal during HD, such as molecular weight, water solubility, and protein affinity. The problem with the physiochemical classification of uremic toxins is that it does not adequately address or reflect the methods of toxin removal from current or contemporary HD techniques (adsorption, convection, and diffusion mechanisms).
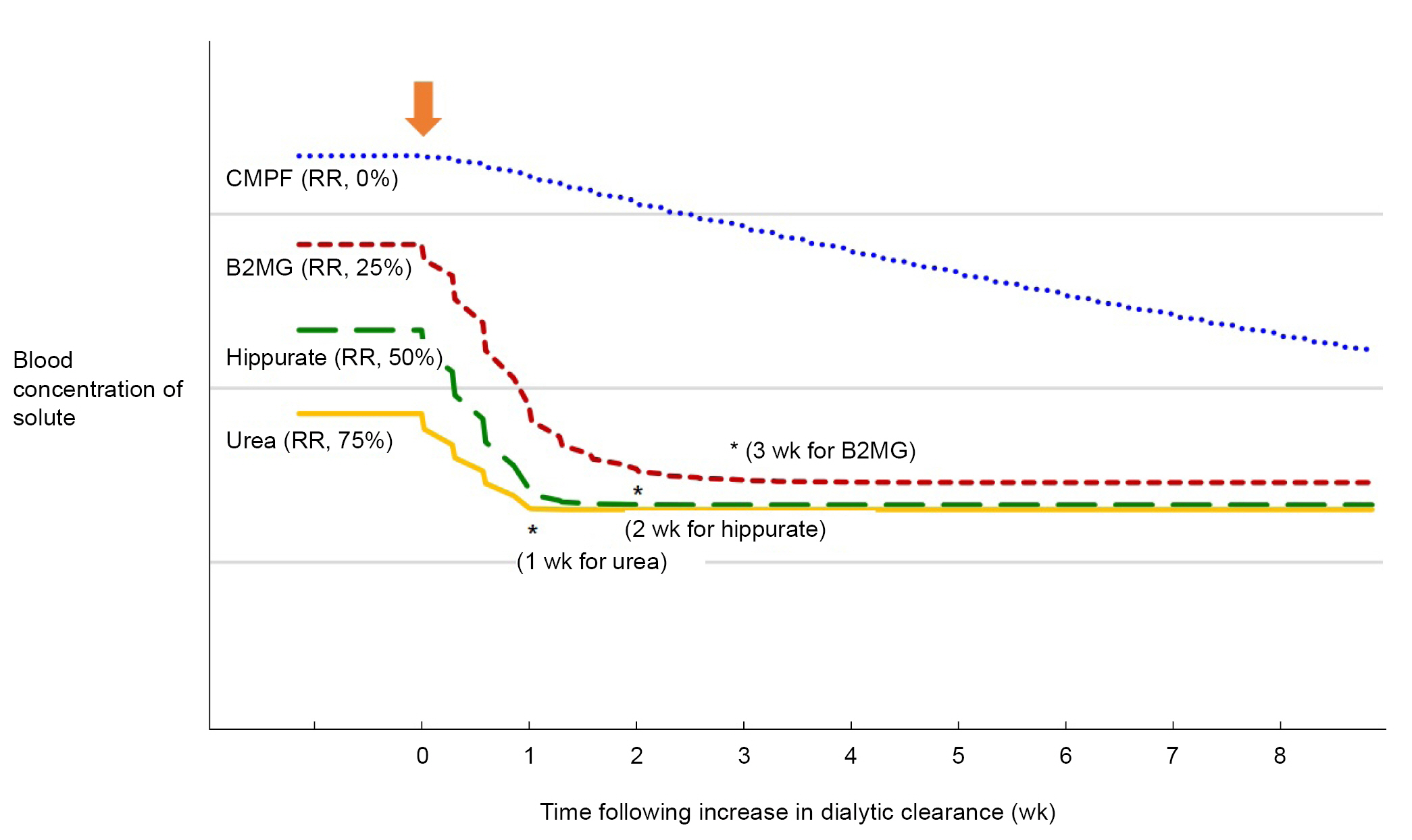
The modeled effect of increasing dialytic clearance on time required to reach solute concentration equilibrium.
Modeling was performed for four hypothetical solutes with varying dialytic RRs (0% for CMPF, 25% for β2-microglobulin, 50% for hippurate, and 75% for urea, respectively) when receiving hemodialysis as a 4-hour thrice-weekly treatment. It was assumed that the intercompartment clearance was higher than the dialytic clearance so that the accessible compartments would rapidly refill from the inaccessible compartments during dialysis; therefore, RR can represent blood, plasma or serum concentration. It was also assumed that each solute was generated constantly and has not nondialytic clearance. The colored lines represent the average concentration of each solute per week. The arrow indicates the time at which dialytic clearance of each solute increases. The asterisks (*) indicate the time at which the concentration for each solute was within 1% of equilibrium during the following week’s dialysis. The solute concentration is plotted as a relative percentage of prehemodialysis concentration on the y-axis, with the weeks following increase in dialytic clearance on the x-axis. Modified from Husain-Syed et al. [8] with permission of Karger.
B2MG, β2-microglobulin; CMPF, 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid; RR, reduction ratio.
To overcome these limitations of EUTox classification, Rosner et al. [1] proposed a new classification system for uremic toxins in 2021. Unlike the EUTox classification, which focuses on the physicochemical properties of uremic toxins, this new classification is characterized by linking uremic toxins with clinical outcomes and quality of life in patients with severe renal failure [1,9]. In this classification, Rosner et al. [1] categorized uremic toxins into two major groups, exogenous and endogenous uremic toxins, and subdivided each according to their molecular characteristics. The clearance of these uremic toxins was also classified according to dialyzer characteristics (Table 1) [1,10]. In addition, Rosner et al. [1] proposed a panel of biomarkers representative of each uremic toxin in this classification: urea for small (<500 Da) water-soluble molecular mass clearance, parathyroid hormone (9.5 kDa) and B2MG (11.8 kDa) for small-middle (0.5−15 kDa) molecular mass clearance, kappa FLC (22.5 kDa) for medium-middle (>15−25 kDa) molecular mass clearance, and lambda FLC (45 kDa) for large-middle (>25−58 kDa) molecular mass clearance. Additionally, IS and pCS have been proposed for the clearance of protein-bound solutes.
In conclusion, several studies, including this, have revealed that uremic toxins, which are not well removed by conventional high-flux HD, are more effectively removed by new dialysis modalities, such as high-volume HDF or MCO-HD. However, it will be necessary to clarify whether the removal of uremic toxins is related to clinical outcomes in future studies.
Notes
Conflicts of interest
The author has no conflicts of interest to declare.
Data sharing statement
The data presented in this study are available on request from the corresponding author.

