Comparison of the medium cutoff dialyzer and postdilution hemodiafiltration on the removal of small and middle molecule uremic toxins
Article information
Abstract
Background
The medium cutoff (MCO) dialyzer increases the removal of several middle molecules more effectively than high-flux hemodialysis (HD). However, comparative data addressing the efficacy and safety of MCO dialyzers vs. postdilution hemodiafiltration (HDF) in Korean patients are lacking.
Methods
Nine patients with chronic HD were included in this pre-post study. Patients underwent HD with an MCO dialyzer for 4 weeks, followed by a 2-week washout period using a high-flux dialyzer to minimize carryover effects, and then turned over to postdilution HDF for 4 weeks. Reduction ratios and differences in the uremic toxins before and after dialysis were calculated from the MCO dialysis, postdilution HDF, and high-flux HD. In the in vitro study, EA.hy926 cells were incubated with dialyzed serum.
Results
Compared to postdilution HDF, the MCO dialyzer achieved significantly higher reduction ratios for larger middle molecules (myoglobin, kappa free light chain [κFLC], and lambda FLC [λFLC]). Similarly, the differences in myoglobin, κFLC, and λFLC concentrations before and after the last dialysis session were significantly greater in MCO dialysis than in postdilution HDF. The expression of Bax and nuclear factor κB was decreased in the serum after dialysis with the MCO dialyzer than with HDF.
Conclusion
Compared with high-volume postdilution HDF, MCO dialysis did not provide greater removal of molecules below 12,000 Da, whereas it was superior in the removal of larger uremic middle molecule toxins in patients with kidney failure. Moreover, these results may be expected to have an anti-apoptotic effect on the human endothelium.
Introduction
Kidney failure is characterized by a progressive loss of elimination capacity, followed by the accumulation of various compounds referred to as uremic toxins [1]. With growing concern about the association of middle molecules or larger low-molecular-weight proteins with increased mortality and morbidity of chronic hemodialysis (HD) patients, new dialysis modalities, including convective therapy, have been developed to remove these uremic toxins [2]. Hemodiafiltration (HDF) provides both effective diffusive clearance of small molecules and superior removal of middle molecules. Previous studies have shown that HDF can reduce intradialytic hypotension, amyloidosis, and accelerated atherosclerosis [3–6]. Moreover, a previous randomized controlled trial supported the superiority of high-convective-volume HDF in reducing all-cause mortality when compared with high-flux HD [7], possibly due to more effective removal of larger uremic retention solutes. However, several meta-analyses did not find a significant difference in the overall mortality between patients treated with HDF and those treated with HD, because some trials were of suboptimal quality and underpowered [8–10].
In this regard, medium cutoff (MCO) dialyzers utilize a novel class of membranes that are designed to increase the removal of larger middle molecules, yet have low permeability for albumin [11]. Specifically, MCO membranes have slightly larger pores and a tighter pore distribution than high-flux membranes [12]. Although a few studies have compared the removal of uremic toxins by MCO dialyzers or high-flux HD [13–16], comparisons between MCO dialyzers and high-convective volume postdilution HDF in terms of the removal of large uremic molecules, particularly free light chains (FLC), and endothelial toxicity in dialysis patients are scarce. In this study, we compared the efficacy of the reduction of middle molecules between MCO dialyzers and high-volume postdilution HDF. Additionally, we performed an in vitro study to determine whether filtered serum obtained after dialysis with an MCO dialyzer was less toxic to EA.hy926 human vascular endothelial cells than serum obtained after high-volume HDF.
Methods
Patients and study design
Maintenance HD patients participated in this prospective, controlled, open-label, nonrandomized, single-center pre-post study. Among HD patients aged ≥20 years, 12 were enrolled in the study. However, three patients dropped out due to withdrawal of consent during the eligibility period. Patients received HD with an MCO dialyzer for 4 weeks, followed by a 2-week washout period using a high-flux dialyzer to minimize carryover effects. Subsequently, the patients turned over to postdilution HDF for 4 weeks. The present study used serum samples collected from patients at the Chonnam National University Hwasun Hospital.
The study protocol was approved by the Institutional Review Board of the Chonnam National University Hwasun Hospital (No. CNUHH-2017-186). All patients provided written informed consent before inclusion in the study.
Dialysis materials and treatment procedures
Patients underwent HD performed with either a Theranova 400 dialyzer (Baxter International Inc.) or an FX 60 or 80 dialyzer (Fresenius Medical Care) according to body surface area. All patients underwent 4 hours of dialysis three times a week using Artis Physio machines (Baxter International Inc.). Online HDF was conducted in a postdilution pressure-controlled mode with a target convective ultrafiltration volume of ≥20 L. The dialysis regimens of each patient, including blood flow, dialysate flow, and treatment duration per session, were not altered.
Determination of small and middle molecules
We measured differences in the clearance of uremic toxins provided by the two treatment options. The uremic toxins were classified into the following types as specified by the European Union Toxin Working Group: small molecules, including blood urea nitrogen (BUN) (60 Da), creatinine (113 Da), and uric acid (168 Da); middle molecules, including β2-microglobulin (β2MG) (11,800 Da), myoglobin (17,800 Da), kappa FLC (κFLC, 25,000 Da), and lambda FLC (λFLC, 50,000 Da); and albumin (66,000 Da).
Clinical outcomes
Baseline clinical information was collected, including age, sex, body weight, height, dialysis vintage, and type of vascular access, and Kt/V was calculated using the second-generation formula for single-pool values to determine the appropriate level of HD for each patient [17]. The efficacy of each dialysis treatment was assessed by calculating the reduction ratios (RRs) and differences in the uremic toxins before and after each MCO dialysis, postdilution HDF, and high-flux HD at the end of the 2- or 4-week treatment period. The RRs were calculated using the following formula:
where Cpre and Cpost are the measured concentrations of the solute before and at the end of the treatments, respectively.
The corrected plasma concentrations of the middle molecules were determined using the following formula [18]:
where BWpre and BWpost are the body weights before and after dialysis, respectively.
In vitro study
Blood samples were obtained from patients at the last dialysis session of the MCO dialysis and postdilution HDF treatment periods, and the serum was separated by centrifugation and transferred to contamination-free bottles. All samples were stored at –80 ℃ until analysis. The EA.hy926 human vascular endothelial cell line (American Type Culture Collection [ATCC]) was cultured in Dulbecco’s modified Eagle’s medium (30-2002, ATCC) at 37 °C in 5% CO2. Heparin (0.6 IU/mL) was added to the incubation medium for all experiments. The cells were incubated with 2.5% serum from patients or 2.5% fetal bovine serum for 16 hours. The final concentration and duration of serum incubation were determined to be based on the appropriate viscosity of the incubation media and the activation of apoptotic proteins (Supplementary Fig. 1; available online).
Western blot analyses and primary antibodies
Western blot analyses were performed as described previously [19]. The cells were harvested, resuspended in lysis buffer, and briefly sonicated. After centrifugation, the supernatant was prepared as a protein extract. Equal concentrations of protein were separated on 8% or 12% sodium dodecyl sulfate-polyacrylamide gels, and the proteins were transferred onto nitrocellulose membranes. Densitometry was performed using Scion Image software (Scion Corporation). The experiments were repeated at least twice. The primary and secondary antibodies used for western blotting are listed in Supplementary Table 1 (available online).
Cell viability test
Cell viability was determined using the CyQUANT MTT cell viability assay kit (V13154; Invitrogen). Absorbance at 570 nm was detected using a 96-well microplate reader (BioTek Instruments). Cell viability was expressed as the fraction of the surviving cells relative to the fetal-bovine-serum-treated cells.
Statistical analyses
The sample size was determined on the basis of the β2MG concentrations. When the effect size was assumed by the mean and standard deviation of differences of β2MG at the level of the in the pre- and postdialysis of both HDF and the MCO dialyzer, eight patients were calculated to provide 80% power at a two-sided alpha level of 0.05 using the Wilcoxon signed-rank test. The expected dropout rate was assumed to be 20%; accordingly, at least 10 patients were required initially. The results were expressed as the median and interquartile range. The statistical significance of differences between the treatments was determined using the Friedman or Wilcoxon signed-rank test. Carryover effects were assessed by comparing the predialysis concentrations of uremic toxins in the first sessions of MCO and HDF. For a more conservative interpretation, p-values of <0.017 (Bonferroni method) were considered statistically significant for multiple comparisons. For the in vitro studies, the statistical significance of differences was determined using an unpaired Student t test or one-way analysis of variance followed by a post hoc Tukey test. All statistical analyses were performed using IBM SPSS version 25.0 (IBM Corp.) and GraphPad Prism version 9.1.2 (GraphPad Software, Inc.).
Results
Baseline characteristics of patients
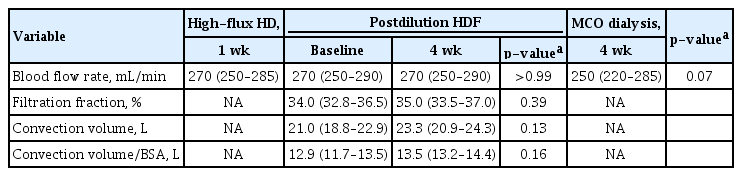
Baseline characteristics are shown in Table 1. The median age of all patients was 58.0 years (interquartile range [IQR], 50.0–69.5 years), and 55.6% were male. The median Kt/V value was 1.58 (IQR, 1.46–1.78). Only one patient underwent dialysis using an arteriovenous graft. The median values of blood flow rate and dialysis vintage were 270 mL/min (IQR, 250–285 mL/min) and 8.8 years (IQR, 6.1–18.6 years), respectively. The changes in blood flow rate did not differ between the high-flux HD, postdilution HDF, and MCO dialysis treatments (p = 0.07) (Table 2). In the first session of online HDF, the median value of the achieved convection volume was 21.0 L/session (IQR, 18.8–22.9 L/session). High convection volume with or without adjustment for body surface area was maintained at the postdilution HDF until the last dialysis session (23.3 L and 13.5 L, respectively) (Table 2). The predialysis concentrations of uremic toxins in serum samples obtained at the first MCO and postdilution HDF dialysis sessions were not significantly different. Therefore, there was no evidence of carryover effects (Supplementary Table 2; available online).
Treatment with the medium cutoff dialyzer increased reduction ratios of small and middle uremic toxins compared to postdilution hemodiafiltration
We compared the pre- and postdialysis serum concentrations of small and middle uremic toxins during the last session of each dialysis modality (Table 3). Of note, BUN, creatinine, uric acid, β2MG, myoglobin, κFLC, and λFLC concentrations were significantly lower after dialysis compared with predialysis measurements regardless of dialysis modality. Differences in pre- and postdialysis concentrations of BUN, creatinine, and uric acid did not differ between high-flux HD, postdilution HDF, and the MCO dialyzer (Table 4); however, differences in the values for β2MG, myoglobin, κFLC, and λFLC were significantly greater after MCO dialysis. In particular, compared with postdilution HDF, differences in myoglobin and λFLC were significantly greater after MCO dialysis.

Comparison of pre- and postdialysis concentrations of uremic toxins in patients who underwent high-flux HD for 4 weeks, then postdilution HDF washout for 2 weeks, and then turned to MCO dialysis for 4 weeks

Differences between pre- and postdialysis concentrations of uremic toxins in patients who underwent high-flux HD for 4 weeks, postdilution HDF washout for 2 weeks, and then turned to MCO dialysis for 4 weeks
Next, we investigated the RRs of small and middle uremic toxins in patients receiving postdilution HDF and MCO dialysis at the last session of each dialysis modality (Fig. 1; Supplementary Table 3, available online).
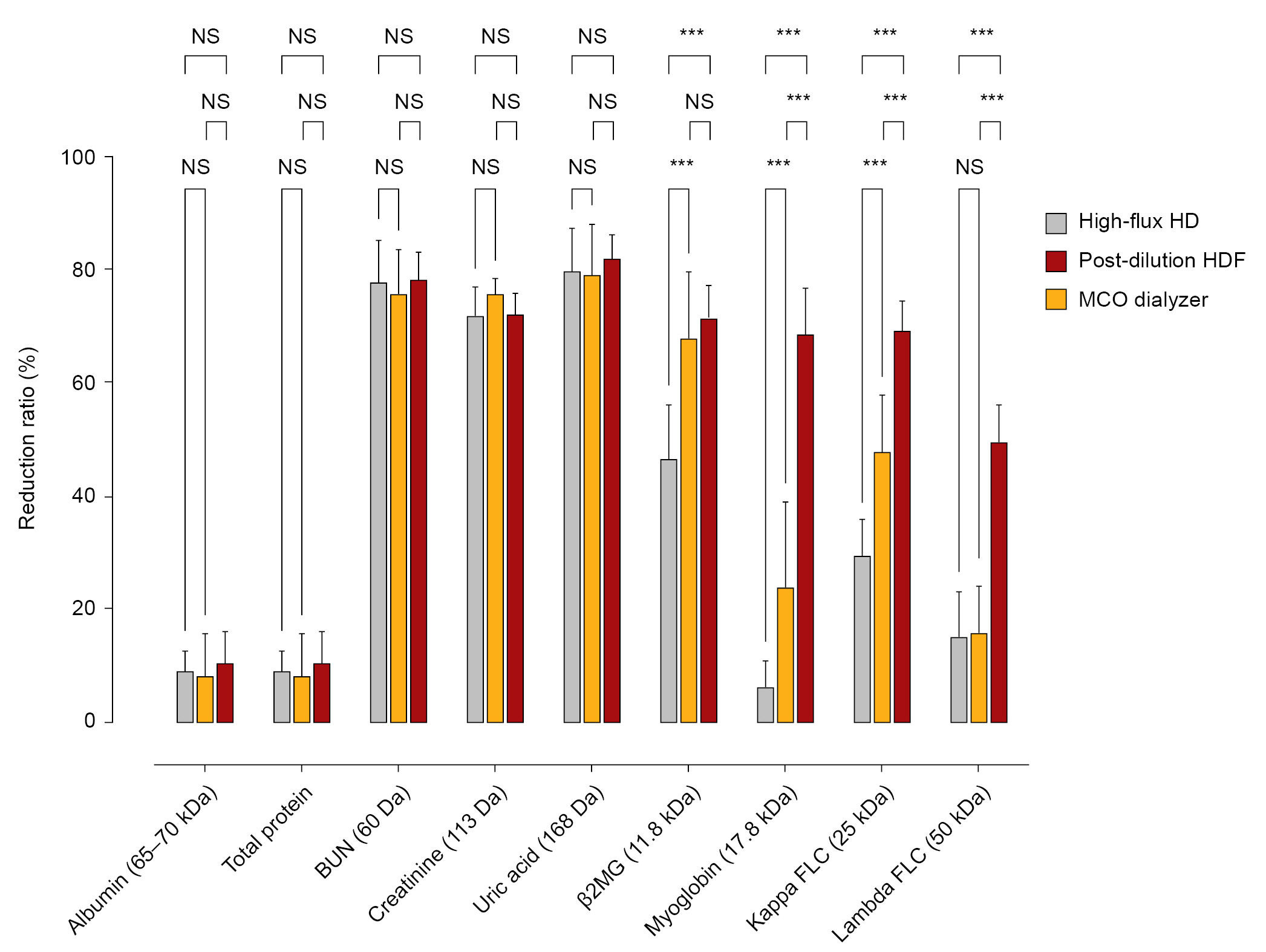
Reduction ratios for small and middle uremic toxins in patients who underwent MCO dialysis for 4 weeks, high-flux HD washout for 2 weeks, and then turned to postdilution HDF for 4 weeks.
BUN, blood urea nitrogen; β2MG, β2-microglobulin; FLC, free light chain; HD, hemodialysis; HDF, hemodiafiltration; MCO, medium cutoff membrane; NS, not statistically significant.
***p < 0.001.
In the post hoc analyses, the RRs of β2MG were not different between the postdilution HDF and MCO dialysis (67.9% ± 11.7% vs. 71.6% ± 5.7%, p = 0.26). However, MCO dialysis resulted in a significantly greater RR for myoglobin, κFLC, and λFLC compared with postdilution HDF. Interestingly, MCO dialysis produced a three-fold greater RR for λFLC compared with postdilution HDF (49.8% ± 6.5% vs. 15.8% ± 8.5%, p = 0.008), while the RRs high-flux HD and postdilution HDF did not differ (15.3% ± 8.0% vs. 15.8% ± 8.5%, p = 0.95). A comparison of albumin loss showed no significant differences between postdilution HDF and MCO dialysis (Table 4; Supplementary Table 3, available online).
Treatment with the medium cutoff dialyzer reduced apoptosis of a human vascular endothelial cell line through activation of the NF-κB signaling pathway
To examine whether the remaining uremic toxins in serum after each dialysis modality exacerbated vascular injury, we performed in vitro studies to explore the effects of purified sera on vascular apoptosis and the nuclear factor κB (NF-κB) signaling pathway (Fig. 2A, B). After incubation of human endothelial cells with serum obtained after MCO dialysis, the ratio of Bax/Bcl-2 expression was lower than after postdilution HDF, as was NF-κB p65 expression. In addition, we found that recovered cell viability in endothelial cells treated with MCO serum compared with serum after postdilution HDF. We added this in the results section (Fig. 2C).

Serum obtained from patients after completing 4 weeks of MCO dialysis and again after 4 weeks of postdilution HDF was added to human vascular endothelial cells (2.5%) for 16 hours, and the expression of Bax, Bcl-2, and NF-κB p65 was assessed.
(A) Western blot analyses of Bax, Bcl-2, and NF-κB p65 protein expression in human endothelial cells incubated with serum after each dialysis modality. (B) Relative protein intensities are presented. The values for control cells incubated with fetal bovine serum were set to 1. (C) Cell viability assay. All values are presented as the mean ± standard error of the mean.
HDF, hemodiafiltration; MCO, medium cutoff membrane; NF-κB, nuclear factor κB; NS, not statistically significant. **p < 0.01; ***p < 0.001.
Discussion
Among the uremic retention compounds, middle molecules or low-molecular-weight proteins with molecular weights ranging from approximately 5,000 to 50,000 Da, including β2MG, myoglobin, FLC, parathyroid hormone, fibroblast growth factor-23, and retinol-binding protein, are considered to have detrimental clinical effects in patients with uremia [20]. Plasma myoglobin concentrations are typically greater in patients with both pre- and postdialysis than in healthy controls [21]. Furthermore, β2MG and FLC have been recognized as surrogate markers for predicting mortality in patients undergoing HD [22,23].
This pre-post study revealed that the MCO dialyzer had a high efficacy for the removal of β2MG, myoglobin, κFLC, and λFLC. Additionally, compared with high convection volume postdilution HDF, the MCO dialyzer treatment showed a greater RR for myoglobin, κFLC, and λFLC.
Previous studies have shown the superiority of the MCO dialyzer over high-flux HD in the reduction of β2MG, κFLC, λFLC, myoglobin, interleukin (IL)-1β, and IL-6 [13–15]. In addition, a previous randomized crossover trial also showed that mRNA expression of the inflammation markers tumor necrosis factor-α and IL-6 were reduced to a greater extent with the MCO dialyzer than with high-flux HD [16]. Similar to our results, a recent multicenter, randomized controlled study in which 86 patients received 24 weeks of treatment with the MCO dialyzer demonstrated a 33% RR of λFLC, while the RR was only 17% when using a similarly sized high-flux dialyzer [24]. However, these results are not surprising when considering the characteristics of an MCO membrane, which has a high-retention onset, and a cutoff value that limits the loss of albumin compared to conventional high-flux membranes [25,26]. Consequently, the MCO dialyzer can provide remarkable convective clearance of medium- to high-molecular-weight solutes while avoiding significant albumin loss.
In terms of the efficacy of middle molecule removal, online HDF can provide combined diffusive and convective transport, resulting in markedly enhanced clearance of middle to large molecules [27]. There are two major dilution techniques in HDF: pre- and postdilution. A recent observational prospective study demonstrated that the MCO dialyzer produced a greater reduction in λFLC compared with predilution online HDF (43.2% vs. 33.0%, respectively) even with a high mean convection volume of 49.9 L/session of HDF [28]. Conversely, there have been conflicting reports of the efficacy of β2MG removal by the MCO dialyzer compared with postdilution HDF, regardless of convection volume [29–32]. Recently, a controlled crossover study showed that there was no significant difference in the RR for β2MG between the MCO dialyzer and a high convection volume of a mean 24.5 L/session postdilution HDF [33]. Consistent with previous studies [21,29,30,33], our results showed no significant differences in the RRs for β2MG after MCO dialysis or postdilution HDF.
There have been few previous comparisons of the efficacy of FLC removal following MCO dialysis or postdilution HDF. Kirsch et al. [32] showed that the RRs of κFLC and λFLC were 72.9% and 48.1%, respectively, when using the MCO AA prototype dialyzer. These results were superior to the removal of λFLC, but not of κFLC, compared to postdilution HDF. Although the RRs of 69.2% and 49.8% for κFLC and λFLC, respectively, for MCO dialysis in our study were similar to previously reported results [29], the MCO dialyzer treatment resulted in a significantly greater RR for both κFLC and λFLC than postdilution HDF (κFLC, 47.8% and λFLC, 15.8%). Based on these results, MCO dialyzers may be a good therapeutic option for dialysis patients with elevated FLC concentrations, such as those with multiple myeloma or amyloidosis.
Myoglobin-induced endothelial dysfunction is linked to oxidative stress, inflammation, and apoptosis [34,35]. In addition, excessive FLC can lead to apoptosis of proximal tubular epithelial cells and induce the activation of NF-κB [36,37]. Herein, we postulated that a low concentration of middle uremic toxins after MCO dialysis, including myoglobin and FLC, could attenuate the apoptosis of endothelial cells in vitro. To support our hypothesis, we assessed the expression of Bax, an apoptotic marker, and NF-κB proteins in human endothelial cells incubated with serum obtained after each dialysis modality. We found that the expression of these proteins was lower when the patient was treated with MCO dialyzed serum, which suggests that dialysis using MCO membranes could prevent uremic toxin-induced endothelial injury. This result is consistent with the findings of a previous study using ingenuity pathway analysis, which suggested that the serum metabolites and proteins after MCO dialysis have properties of increased proliferation and decreased apoptosis in endothelial cells compared to those after high-flux HD [38].
To the best of our knowledge, this is the first pre-post study assessing the RRs of middle molecules, including FLC, after treatment with a commercial MCO dialyzer, and comparing the results with those produced after treatment using high convection volume postdilution HDF. However, this study had several limitations. First, the study duration was relatively short. Second, the sample size was small; however, this study was conducted with an adequate number of patients to meet the desired statistical power. Lastly, the enrolled patients were not randomly assigned to the MCO dialyzer or the HDF.
In conclusion, this study showed that, compared with high-volume postdilution HDF, dialysis using the MCO dialyzer was superior in terms of the reduction of myoglobin, κFLC, and λFLC in Korean patients, whereas there were no differences in the removal of molecules below 12,000 Da and serum albumin. Moreover, these results may be expected to have an anti-apoptotic effect on the human endothelium. Further long-term prospective studies with large sample sizes are needed to clarify the clinical implications of the substantial reduction of middle molecules using the MCO dialyzer.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.21.287).
Notes
Conflicts of interest
Eun Hui Bae and Soo Wan Kim are the Associate Editors of Kidney Research and Clinical Practice and were not involved in the review process of this article. All authors have no other conflicts of interest to declare.
Funding
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (MSIT) of the Korean government (NRF-2019R1A2C2086276), by the Basic Science Research Program of the NRF of Korea funded by the Ministry of Education (NRF-2018R1D1A1B07042999), and by a grant (BCRI22080) from Chonnam National University Hospital Biomedical Research Institute.
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Author contributions
Conceptualization, Data curation, Formal analysis, Funding acquisition: CSK, SWK
Methodology: CSK, SYJ
Supervision: HSC, EHB, SKM, SWK
Writing – original draft: CSK
Writing – review & editing: All authors
All authors read and approved the final manuscript.