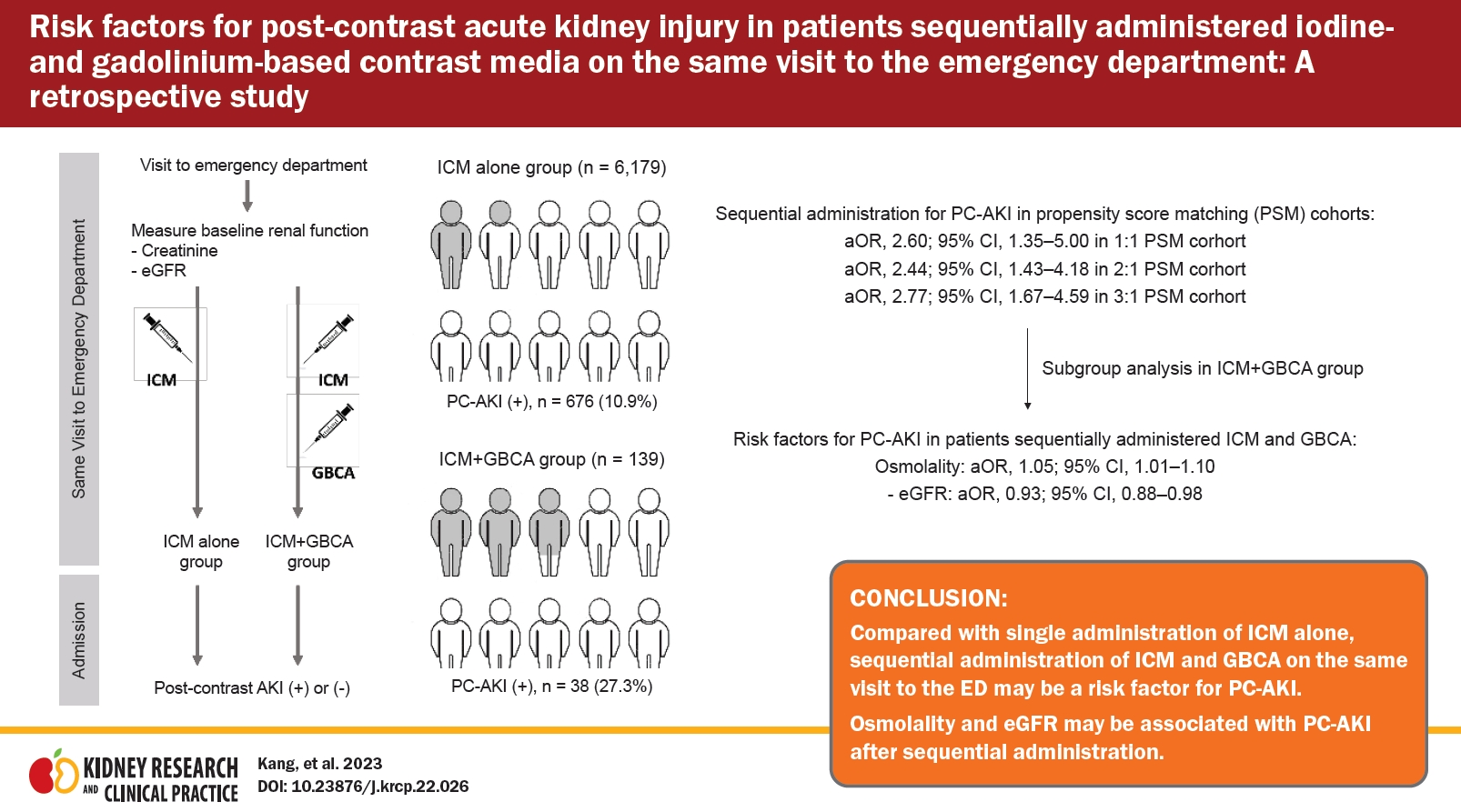
Risk factors for post-contrast acute kidney injury in patients sequentially administered iodine- and gadolinium-based contrast media on the same visit to the emergency department: a retrospective study
Article information
Abstract
Background
This study compares the incidence of post-contrast acute kidney injury (PC-AKI) in patients who received a single administration of iodine-based contrast medium (ICM) with that in patients who received a sequential administration of ICM and gadolinium-based contrast agents (GBCA) in a single visit to an emergency department (ED) to determine the risk factors for PC-AKI.
Methods
Patients who received one or more contrast media in the ED from 2016 to 2021 were included in this retrospective study. They were divided into the ICM alone and ICM + GBCA groups, and the incidence of PC-AKI was compared between the groups. The risk factors were assessed using a multivariable analysis after propensity score matching (PSM).
Results
Overall, 6,318 patients were analyzed, of whom 139 were in the ICM + GBCA group. The incidence of PC-AKI was significantly higher in the ICM + GBCA group than in the ICM alone group (10.9% vs. 27.3%, p < 0.001). In the multivariable analysis, sequential administration was a risk factor for PC-AKI, and single administration was not (adjusted odds ratio [95% confidence interval] in the 1:1, 2:1, and 3:1 PSM cohorts: 2.38 [1.25–4.55], 2.13 [1.26–3.60], and 2.28 [1.39–3.72], respectively). In subgroup analyses of the ICM + GBCA group, osmolality (1.05 [1.01–1.10]) and estimated glomerular filtration rate (eGFR, 0.93 [0.88–0.98]) were associated with PC-AKI.
Conclusion
Compared with a single administration of ICM alone, sequential administration of ICM and GBCA during a single ED visit might be a risk factor for PC-AKI. Osmolality and eGFR might be associated with PC-AKI after sequential administration.
Introduction
Contrast media are indispensable for enhanced imaging examinations, such as computed tomography (CT) and magnetic resonance imaging (MRI), because they allow physicians to gain essential information. Despite their advantages, intravenous iodine-based contrast medium (ICM) and gadolinium-based contrast agents (GBCA) have been identified as causes of post-contrast acute kidney injury (PC-AKI) [1–3], though well-designed meta-analysis studies have reported that the nephrotoxicity associated with these contrast media has been overestimated [4–7].
Nonetheless, multiple administrations of contrast medium in a short period are still proposed as a risk factor for PC-AKI [8]. Because the association between sequential administrations of ICM and GBCA on the same day and the development of PC-AKI is not yet clear, the current guideline from the European Society of Urogenital Radiology recommends that patients with normal or moderately reduced renal function (estimated glomerular filtration rate [eGFR] of >30 mL/min/1.73 m2) should have an interval of at least 4 hours between administrations of ICM and GBCA, based on their half-lives for excretion from the body [9]. On the other hand, the American College of Radiology (ACR) guideline regards that as ambiguous and does not endorse a specific time interval for sequential administrations of two contrast media [10,11].
The currently available evidence about risk factors for PC-AKI, particularly in patients who sequentially receive ICM and GBCA on a single visit to an emergency department (ED), is still limited because this issue is uncommon in a general clinical environment. However, urgent or emergency medical issues can lead to this clinical situation, especially in an ED. Therefore, we examined the incidence of PC-AKI in patients with a baseline eGFR of >30 mL/min/1.73 m2 and compared those who received a single administration of ICM alone with those who received sequential administrations of ICM and GBCA during a single ED visit. We also investigated the risk factors for developing PC-AKI among patients who received sequential administrations of ICM and GBCA during a single ED visit.
Methods
Study design and population
This was a single-center, retrospective cohort study conducted by reviewing data extracted from electronic medical records of patients who visited the ED of Chungnam National University Hospital; approximately 56,000 patients visit the ED annually. This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Chungnam National University Hospital (No. 2021-11-024). The extracted data included only clinical data; no personally identifiable information was collected. Therefore, the need for informed consent was waived.
Patients who were admitted from the ED after either a single administration of ICM or sequential administration of both contrast media were included in this study. Among them, pediatric patients (aged <18 years) and patients who had an eGFR of <30 mL/min/1.73 m2, as measured in the ED; had a medical history of kidney transplantation; or were missing data for creatinine or eGFR measured before the administration of contrast medium were excluded. The enrolled patients were divided into two groups: ICM alone (single administration of ICM in the ED) and ICM + GBCA (sequential administration of ICM and GBCA).
Data from July 2016 to July 2021 were extracted by an experienced research assistant who underwent rigorous training on our explicit protocol, including clearly defined variables and standardized coding methods. The systematic data abstraction was performed by abstractors who were blinded to the overall goals of the research to ensure unbiased chart reviews. Any conflicting or ambiguous charts were flagged by the abstractors for additional review by two board-certified emergency physicians and nephrologists. We recorded the following data from the index visit: baseline demographics (age, body mass index [BMI], sex), Charlson comorbidity index (CCI) calculated from preexisting illness, ED chief complaint, ED disposition, ED vital signs, laboratory results in ED, type and interval of each enhanced image examination in the cohort (both CT and MRI) performed in the ED on the same day, and mortality rates.
Interventions (process of radiologic examinations)
The enhanced CT and MRI procedures included a chest CT, abdominal CT, three-phase CT, brain CT angiography, head and neck CT, neck CT angiography, extremity CT angiography, head MRI with magnetic resonance angiography, spine MRI, and perfusion brain MRI. All CT and MRI scans were performed using a 64-channel system (Somatom Sensation 64; Siemens Healthineers) and a 3T scanner (Achieva 3T; Philips Healthcare), respectively. According to our institutional policy, preventive hydration with a fixed volume (500 mL) of normal saline was infused into patients at high risk of developing PC-AKI after the administration of contrast medium (serum creatinine level of >1.7 mg/dL or eGFR of <45 mL/min/1.73 m2) at the discretion of the attending physicians.
All administrations of contrast medium (Supplementary Table 1, available online) were performed according to institutional protocols (available online at https://www.ctisus.com/protocols). The ICM used in the ED is a low-osmolality and non-ionic contrast agent that was administered intravenously according to image examination-specific protocols at a volume of 80 to 120 mL. Similarly, GBCA was administered at 0.1 mL/kg according to examination-specific protocols.
Outcomes
The primary outcome of this study was the development of PC-AKI. Traditionally, PC-AKI has been defined as a significant increase in serum creatinine from baseline within 72 hours after the last administration of contrast medium [9]. Recently, however, eGFR has gained attention as a potentially better marker of PC-AKI risk [12,13] because it predicts the true GFR more accurately than serum creatinine [14]. Therefore, we added an eGFR-based criterion and defined PC-AKI in this study as an increase in serum creatinine of ≥25% or 0.5 mg/dL over the baseline value [9] or the reduction of eGFR by ≥25% of the baseline value within 72 hours after the last administration of contrast medium (ICM or GBCA) [15]. eGFR was estimated according to the guidelines of the Korean Society of Nephrology [16]. The secondary outcome was AKI recovery, defined as a return to the baseline serum creatinine level, within 7 days after the ED visit [17].
Statistical analysis
Categorical and continuous variables had a non-normal distribution in this study, so differences between the groups were analyzed using the chi-square test with continuity correction in 2 × 2 tables or Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables, with the results expressed as a frequency with the percentile and median values with interquartile ranges (IQRs), respectively.
We performed propensity score matching (PSM) between the ICM alone and ICM + GBCA groups to balance potential covariables. A binary logistic regression model was used to determine the propensity scores for the ICM + GBCA group using baseline characteristics and clinical status in the ED. For the PSM analysis, each patient in the ICM + GBCA group was matched to one patient in the ICM alone group to the nearest fifth decimal point using a nearest-neighbor algorithm. A caliper setting of 0.2 was used. Standardized differences (SDs) were used to confirm a balanced matching result. The matching result was considered balanced when the SD was <0.1. There was no overlapping of non-exposure cases in the final models. Many subjects received ICM alone; therefore, we also used many-to-one PSM (1:1, 2:1, and 3:1) to minimize the standard error between the groups. After PSM, multivariable logistic regression analyses were performed for each PSM cohort. Adjusted odds ratios (aORs) with 95% confidence intervals (CIs) for the exposure variable were calculated in each analysis. Backward selection was used to develop the final adjusted model. The goodness of fit of the final model was evaluated using the Hosmer-Lemeshow test. The results of the logistic regression analysis are expressed as aORs with 95% CIs.
A subgroup multivariable logistic regression analysis was performed to identify the independent risk factors for PC-AKI in the ICM + GBCA group. All variables with a p-value of <0.1 in the univariable analyses were included using the same multivariable logistic regression method just described. Potential multicollinearity was assessed using tolerance and the variance inflation factor (VIF) to verify that multicollinearity did not significantly influence the model’s coefficients. Multicollinearity between variables was defined as a tolerance of <0.1 or a VIF of >10 [18]. Receiver-operating characteristic (ROC) curves were constructed to determine the predictive value of factors independently associated with the development of PC-AKI, and the area under the ROC curve (AUROC) was obtained for single effective variables and their combination. The combination was divided into two steps: first, a probability value was obtained using a binary logistic regression analysis; second, an ROC curve analysis was performed using this probability value as a test variable. The analysis was performed using R software (version 4.1.0; R Foundation for Statistical Computing) and MedCalc version 15.2.2 (MedCalc Software). Results were considered statistically significant at a p-value of <0.05.
Results
Study population
Of the 13,839 patients who received ICM for enhanced CT in the ED, 349 pediatric patients, 64 patients for whom the data for estimating renal function before the use of contrast medium were unavailable, 6,974 patients whose eGFR was <30 mL/min/1.73 m2 at baseline, and 134 patients who had undergone kidney transplantation were excluded (Fig. 1). Of the remaining 6,318 patients, 6,179 (97.8%) and 139 (2.2%) were in the ICM alone and ICM + GBCA groups, respectively.

Flow diagram of the patients included in this study.
CKD, chronic kidney disease; ED, emergency department; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; GBCA, gadolinium-based contrast agents; HD, hemodialysis; ICM, iodine-based contrast medium; PD, peritoneal dialysis.
The demographic and baseline characteristics of the total cohort in this study are provided in Table 1. The groups differed significantly in liver disease (335 [5.4%] vs. 18 [12.9%], p < 0.001) and ischemic heart disease (417 [6.7%] vs. 22 [15.8%], p < 0.001) as past medical history; however, the CCI did not differ between the groups (3 [IQR, 1–4] vs. 3 [IQR, 1–5], p = 0.14) (Table 1). Neurologic symptoms (675 [10.9%] vs. 47 [33.8%], p < 0.001) and cardiac arrest (54 [0.9%] vs. 7 [5.0%], p < 0.001) as the chief complaint during the ED visit were significantly higher in the ICM + GBCA group than in the ICM alone group (Table 1). After performing PSM to adjust the balance between the groups, the baseline characteristics and clinical status of the two groups were as displayed in Table 2, and the adjustment status after PSM that we estimated using the standard mean differences between the groups is shown in Supplementary Fig. 1 (available online). None of the baseline characteristics or clinical status in the ED differed significantly between the groups after PSM (Table 2).
Incidence of post-contrast acute kidney injury and the renal recovery rate within 7 days after the emergency department visit
The ICM + GBCA group developed PC-AKI more frequently than the ICM alone group (676 [10.9%] vs. 49 [35.3%], p < 0.001) (Table 1), but the rate of renal recovery did not differ significantly between the groups (501 [74.1%] vs. 30 [78.9%], p = 0.57) (Table 1). The incidence of PC-AKI remained significantly higher in the ICM + GBCA group than the ICM alone group in all PSM cohorts (Table 2).
Risk factors for developing post-contrast acute kidney injury in all propensity-matched cohorts
The multivariable logistic regression analysis for each PSM cohort is provided in Table 3. Sequential administration of ICM and GBCA, compared with a single administration of ICM, was independently associated with the development of PC-AKI in all PSM cohorts (Table 3).
Subgroup analyses in the iodine contrast medium + gadolinium-based contrast medium group to investigate the risk factors for developing post-contrast acute kidney injury
The demographic and baseline characteristics of subgroups of patients who received sequential administrations of ICM and GBCA are provided in Supplementary Table 2 (available online). The PC-AKI group showed significantly higher osmolality (303.0 mOsmol/kg [IQR, 295.0–316.5] vs. 291.0 mOsmol/kg [IQR, 283.0–305.1], p < 0.01), sodium (142.1 mEq/L [IQR, 138.9–146.3] vs. 138.8 mEq/L [IQR, 136.2–140.8], p < 0.01), chloride (106.2 mEq/L [IQR, 98.0–110.5] vs. 103.3 mEq/L [IQR, 99.0–108.1], p = 0.04), and glucose (152.0 mg/dL [IQR, 110.8–227.0] vs. 114.0 mg/dL [IQR, 92.0–154.5], p < 0.01) and lower platelet counts (180.0 × 103/μL [IQR, 130.8–247.5] vs. 226.0 × 103/μL [IQR, 167.5–281.5], p = 0.03) than the non-PC-AKI group. In addition, the time interval between the administrations of ICM and GBCA (<4 or ≥4 hours) in the PC-AKI group was significantly shorter than in the non-PC-AKI group (4.6 hours [IQR, 2.2–8.0] vs. 1.8 hours [IQR, 1.0–3.4], p < 0.01).
In our assessment for potential multicollinearity, sodium and glucose did not show significant collinearity with serum osmolality (sodium [tolerance, 0.818; VIF, 1.223] and glucose [tolerance, 0.919; VIF, 1.089]); however, they were excluded from the multivariable analysis because unrecognized collinearity was strongly expected between them and serum osmolality. In the multivariable analysis, the following variables were adjusted: serum osmolality, whether or not the time between the administration of the contrast media was >4 hours, platelet count, creatinine level, eGFR, and chloride level. The multivariable analysis revealed that osmolality (aOR, 1.05 [95% CI, 1.01–1.10]; p = 0.02) and eGFR (aOR, 0.93 [95% CI, 0.88–0.98]; p = 0.01) were independently associated with the development of PC-AKI, whereas the time interval between contrast administrations was not (Table 4).
Subgroup analysis to find the predictive performance of osmolality and estimated glomerular filtration rate for post-contrast acute kidney injury development
Fig. 2 shows the predictive performance of serum osmolality and eGFR when examined independently and in combination. The AUROC values for serum osmolality and eGFR were 0.701 (95% CI, 0.592–0.796) and 0.613 (95% CI, 0.503–0.716), respectively (Fig. 2). Their corresponding cut-off values, sensitivity, and specificity were as follows: 292 mOsmol/kg, 87.1% (95% CI, 70.2–96.4), and 50.0 (95% CI, 36.1–63.9), respectively, for osmolality; 78 mL/min/1.73 m2, 58.3% (95% CI, 36.6–77.9), and 66.7% (95% CI, 53.7–78.0), respectively, for eGFR (Fig. 2). The AUROC values for the development of PC-AKI were numerically higher when the two variables (osmolality and eGFR) were used in combination than when they were used alone (AUROC, 0.714 [95% CI, 0.578–0.825]) (Fig. 2).
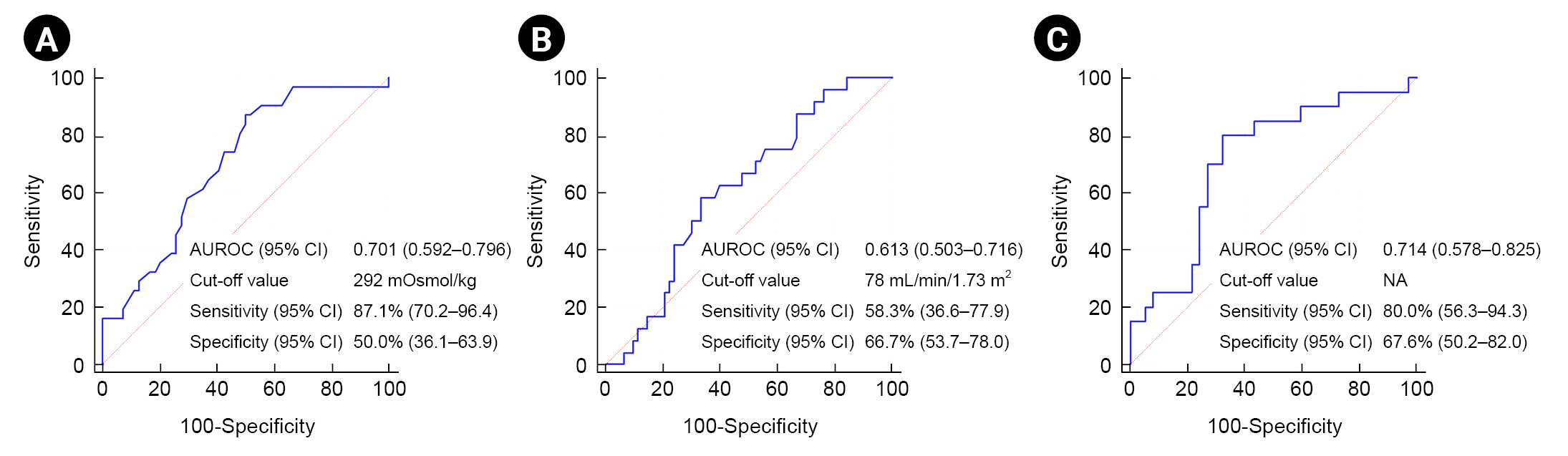
Predictive performance of serum osmolality (A), eGFR (B), and their combination (C) on the development of PC-AKI in patients who received sequential administration of ICM and GBCA. (A)
AUROC, area under the receiver-operating characteristic curve; CI, confidence interval; eGFR, estimated glomerular filtration rate; GBCA, gadolinium-based contrast agents; ICM, iodine-based contrast media; NA, not applicable; PC-AKI, post-contrast acute kidney injury.
Discussion
In this study, the incidence rates for PC-AKI after the administration of contrast media were 10.9% and 27.3% in the ICM alone and ICM + GBCA groups, respectively. This finding is in line with the results of previous studies that the rate of overall contrast-induced nephropathy was 10.6% in patients who received ICM alone [19,20], and the incidence of AKI was higher after combined use of ICM and GBCA than after the use of a single agent [21]. Furthermore, we found that the sequential administration of ICM and GBCA during a single ED visit for sequential radiologic examinations increased the risk of PC-AKI compared with a single administration of ICM alone. We investigated the risk factors for the development of PC-AKI using subgroup analyses of the ICM + GBCA group. Notably, a time interval of 4 hours between contrast administrations, as suggested in a current guideline [9], was not independently associated with the development of PC-AKI, whereas serum osmolality and eGFR were independently associated with the development of PC-AKI. Although urgent medical issues that might require sequential administrations of ICM and GBCA are generally uncommon, they do occur, and ED physicians in charge of resuscitation occasionally encounter this circumstance. Therefore, we suggest that ED physicians consider the effects of serum osmolality and reduced kidney function in urgent medical circumstances that require them to perform sequential administration of ICM and GBCA in the ED.
The findings of this study are contrary to those of a previous study that examined the association between sequential administration of contrast media and the development of PC-AKI [22]. The discrepancy can be attributed to our use of eGFR as a marker of PC-AKI in this study, whereas the other study evaluated the development of PC-AKI using only the serum creatinine level [22]. Several studies have shown that GBCA induces kidney injury in several hours [23,24], in addition to causing a significant decrease in the eGFR [25,26]. These factors could explain why we found a higher incidence of PC-AKI after sequential administration of ICM and GBCA than in the previous study [22]. Although the equations for calculating eGFR can induce measurement errors and augment the clearance rate, often resulting in overestimation, it is commonly used in AKI screening to predict the risk status [27–29]. Furthermore, one previous study stated that the serum creatinine level increases immediately after a decrease in the eGFR in the presence of AKI, making creatinine a suboptimal indicator of renal function in patients with AKI [30]. Consequently, a recent randomized prospective study for the development of PC-AKI after the administration of contrast media used eGFR as an additional marker of AKI [15]. In addition, GBCA, which is a high-osmolar contrast medium, can induce intense and prolonged vasoconstriction at the corticomedullary junction of the kidney and directly impair the autoregulatory ability of the kidney by causing a decline in nitric oxide production [31,32]. In line with that pathophysiologic pathway, our subgroup analysis showed that high serum osmolality and low eGFR at baseline were independently associated with the development of PC-AKI. It appears that ICM and GBCA synergistically interact with osmolar and renal function status, causing a rapid decline in eGFR and the development of PC-AKI.
We found that low eGFR was independently associated with the development of PC-AKI in patients who sequentially received ICM and GBCA during the same ED visit. Low eGFR is widely accepted as an independent risk factor for the development of PC-AKI induced by ICM or GBCA [33,34]. McDonald et al. [34] found that eGFR of ≤45 mL/min/1.73 m2 before an administration of ICM increased the risk of renal replacement therapy. However, the cut-off value for eGFR associated with the development of PC-AKI in patients who sequentially received ICM and GBCA within a short interval remains unclear. In our results, the eGFR cut-off value for predicting the development of PC-AKI was 78 mL/min/1.73 m2, which suggests that the optimal safe eGFR in patients who sequentially receive ICM and GBCA within a short interval could be higher than that in patients who receive a single administration of contrast medium. In other words, PC-AKI can develop even in patients with normal kidney function (eGFR of >60 mL/min/1.73 m2) after sequential administrations of two contrast media. However, the clinical relevance of that finding is limited by our study design. Therefore, we emphasize that a further study is needed to investigate an optimal eGFR cut-off value for patients who receive sequential administrations of ICM and GBCA and develop preventive strategies for patients whose eGFR is lower than that in an ED setting.
High serum osmolality was independently associated with the development of PC-AKI in patients who sequentially received ICM and GBCA on the same ED visit. This finding is in line with previous studies that commonly revealed that high serum osmolality was associated with the development of PC-AKI after an administration of ICM [19,35]. Serum osmolality is widely accepted as a typical indicator representing body fluid balance, with a high osmolality linked to dehydration [36,37]. The ACR guideline clearly states that dehydration is an important risk factor for PC-AKI, and thus the major preventive action to mitigate the risk of PC-AKI is to provide intravenous volume expansion using 0.9% normal saline prior to ICM administration [38]. Serum osmolality numerically improved the predictive performance of our model when it was used in combination with eGFR, compared with using eGFR alone (AUROC, 0.613 [95% CI, 0.503–0.716] to 0.714 [95% CI, 0.578–0.825]). Given previous findings of a significant association between high osmolality and the development of PC-AKI in patients who require sequential administrations of two contrast media on the same ED visit, we suggest that high serum osmolality is likely to be a risk factor for the development of PC-AKI. Furthermore, we suggest that a strategy should be developed to administer preventive hydration based on serum osmolality and/or eGFR prior to sequential treatment with both contrast media.
This study has several limitations. First, because it was a retrospective study, we can report only associations and not causation. Furthermore, like all retrospective studies, this one contains inherent selection bias. We were aware of the possible biases and held multiple meetings to ensure that the patients were correctly identified and the data collection protocol was suitably standardized; adjustment for comorbidities was also made to reduce bias. To reduce selection bias and simulate a randomized controlled trial, the PSM method was used in this study. In our PSM cohort, both groups showed a well-balanced distribution of demographics and most confounders. However, age, CCI, and eGFR were not balanced in the 1:1 PSM cohort, and thus unmeasured or unmeasurable confounders might still remain. Therefore, a further study with prospectively collected data from a large sample is needed to confirm our results. Second, several nephrotoxic medications administered during the hospital stay were not used in the entire study population, which created bias in confirming the effect of sequential administrations of contrast media on the development of PC-AKI. However, we minimized the non-estimated bias in our subgroup analysis to investigate independent risk factors for the development of PC-AKI in patients who sequentially received ICM and GBCA. Third, our institution's policy for preventive hydration in patients at high risk of developing PC-AKI is to leave the decision completely to the physician discretion, and thus it is not clear whether it was applied to all patients. However, we confirmed that all included patients at high risk of developing PC-AKI underwent preventive hydration in the form of a fixed volume (500 mL) of normal saline by thoroughly reviewing medical records kept by physicians and nurses. Fourth, nephrogenic systemic fibrosis (NSF), which is a major concern for GBCA nephrotoxicity, was not estimated in this study. A diagnosis of NSF is usually made with a detailed patient history, thorough clinical examination, and the identification of characteristic findings a few weeks or months after an administration of GBCA [36]. Therefore, a further study in the ward or intensive care unit setting is required to confirm how combining two contrast media affects the incidence of NSF. Fifth, because our study sample was small, serum osmolality and eGFR could not be included in the multivariable analysis as categorical variables (osmolality, hypoosmolality vs. normal vs. hyperosmolality; eGFR, normal vs. mild kidney dysfunction vs. moderate kidney dysfunction). Therefore, a multicenter study with a large cohort is required to enhance the generalizability of our results and their easy application in a clinical environment.
Compared with a single administration of ICM alone, sequential administration of ICM and GBCA during a single ED visit could be a risk factor for the development of PC-AKI in patients with an eGFR of >30 mL/min/1.73 m2. Baseline osmolality and eGFR might be independently associated with the development of PC-AKI after sequential administration of ICM and GBCA. Well-designed prospective studies are needed to investigate the risk factors for PC-AKI and develop ED setting–specific preventive strategies.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2021R1F1A1050858).
Data sharing statement
The data presented here are available from the corresponding author upon request. The data are not publicly available because of ethical concerns.
Authors’ contributions
Conceptualization: CK
Formal analysis: JSP, DEC
Funding acquisition: DEC
Investigation: SSH, DEC
Supervision: JSP
Writing–original draft: CK, SSH
Writing–review & editing: All authors
All authors read and approved the final manuscript.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.22.026).