Histologic evaluation of activity and chronicity of lupus nephritis and its clinical significance
Article information
Abstract
The National Institutes of Health (NIH) lupus nephritis activity and chronicity indices, which comprise six activity scores and four chronicity scores, have a long development history. The 2018 revised International Society of Nephrology/Renal Pathology Society classification for lupus nephritis adopted the most recent NIH indices to replace subclasses A, C, and A/C. Although an evidence-based approach should further evaluate the clinical significance of the modified NIH indices, recent validation studies demonstrated that the modified chronicity indices have a strong correlation with kidney outcome of lupus nephritis.
Introduction
Lupus nephritis is a renal manifestation of systemic lupus erythematosus (SLE) that displays a broad spectrum of histologic changes. The International Society of Nephrology/Renal Pathology Society (ISN/RPS) lupus nephritis classification is the most widely used system for categorizing glomerular lesions of lupus nephritis according to the location of immune complex deposition [1,2]. Since its creation in 2004, improvements in the classification system have been discussed [3], and proposed revisions were published in 2018 [4], such as phase 1 and phase 2 recommendations, which were based on both already published evidence and those lesions needing further studies, respectively. The phase 1 recommendations include refined definitions and newly adopted or refined terminology, such as lesions of mesangial hypercellularity, endocapillary hypercellularity, fibrous or fibrocellular crescents, adhesion, and fibrinoid necrosis. The elimination of the subclasses for class IV (class IV–S and class IV–G) was also proposed because of the uncertain clinical significance and unclear parameters for distinguishing them, which could result in interobserver variability. Regarding disease activity, the authors recommended that the original ISN/RPS designations of activity and chronicity using subclasses A, C, and A/C should be replaced by the modified National Institutes of Health (NIH) lupus nephritis activity and chronicity scoring system (NIH indices) that originated in the previous World Health Organization classification.
This review focuses on pathological evaluation of lupus nephritis disease activity including the history of these NIH indices and their clinical significance, the recent revision of the ISN/RPS classification, and the results of validation studies on the classification’s use. Other approaches to disease activity assessment using histologic evaluation will also be discussed.
Development of the National Institutes of Health indices
Concepts regarding active and chronic lesions in lupus-related kidney lesions and their inclusion into lists can be found in the literature as early as 1979 in an NIH conference report on SLE [5]. In this report, parameters for the activity index (AI) included four glomerular lesions and one tubulointerstitial lesion, and the parameters for the chronicity index (CI) included two glomerular lesions and two tubulointerstitial lesions. Each parameter was scored from 0 to 3+. The original NIH indices, which are similar to current NIH indices, were proposed in research articles by Austin et al. in 1983 [6] and 1984 [7]. In pursuit of histologic predictors of kidney failure in cases of lesions then classified as “diffuse proliferative” or “membranoproliferative” lupus nephritis, the authors adopted these previously defined parameters [5] and developed a semiquantitative scoring system for both active and chronic lesions. Of note, in older studies, the term focal or diffuse “proliferative” lupus nephritis was often used. It is now recognized that these hypercellular lesions include mostly infiltrating inflammatory cells, and thus the term “endocapillary hypercellularity” is now used to describe these active lesions seen in class III or class IV lupus nephritis. Parameters listed for active lesions included glomerular cell proliferation, which corresponds to endocapillary hypercellularity, leukocyte exudation, karyorrhexis/fibrinoid necrosis, presence of cellular crescents, extent of so-called “hyaline” deposits, and degree of interstitial inflammation (Fig. 1). Of note, the term “hyaline” deposits or “hyaline thrombi” was used to describe large, glassy eosinophilic, usually subendothelial, deposits seen by light microscopy, protruding into the capillary lumen. This term is misleading because the deposits are not truly within capillary lumina and do not consist of fibrin, and thus are not true thrombi. All parameters were scored from 0 to 3+, according to the percentage of affected glomeruli (<25%, 1+; 25%–50%, 2+; and >50%, 3+), for an evaluation of glomerular cell proliferation, karyorrhexis/fibrinoid necrosis, and presence of cellular crescents. Scoring according to degree—mild/few (1+), moderate (2+), and severe/extensive (3+)—was applied to the evaluation of leukocyte exudation, hyaline deposits, and interstitial inflammation [7]. Glomerular sclerosis (both global and segmental), presence of fibrous crescents, tubular atrophy, and interstitial fibrosis were listed as parameters of chronic lesions and scored in a similar way to those of active lesions; the percentage of affected glomeruli (for glomerular sclerosis and fibrous crescents) or mild, moderate, and severe changes (for tubular atrophy and interstitial fibrosis) [7]. The karyorrhexis/fibrinoid necrosis and cellular crescents scores were weighted two-fold because these lesions were listed as particularly active parameters in previous studies [8,9]. Therefore, the maximum scores were 24 for active lesions (AI) and 12 for chronic lesions (CI) [7]. This scoring system has been adopted by other studies of histologic evaluation of lupus nephritis [10–14] and is currently included in most major renal pathology textbooks [15–18].
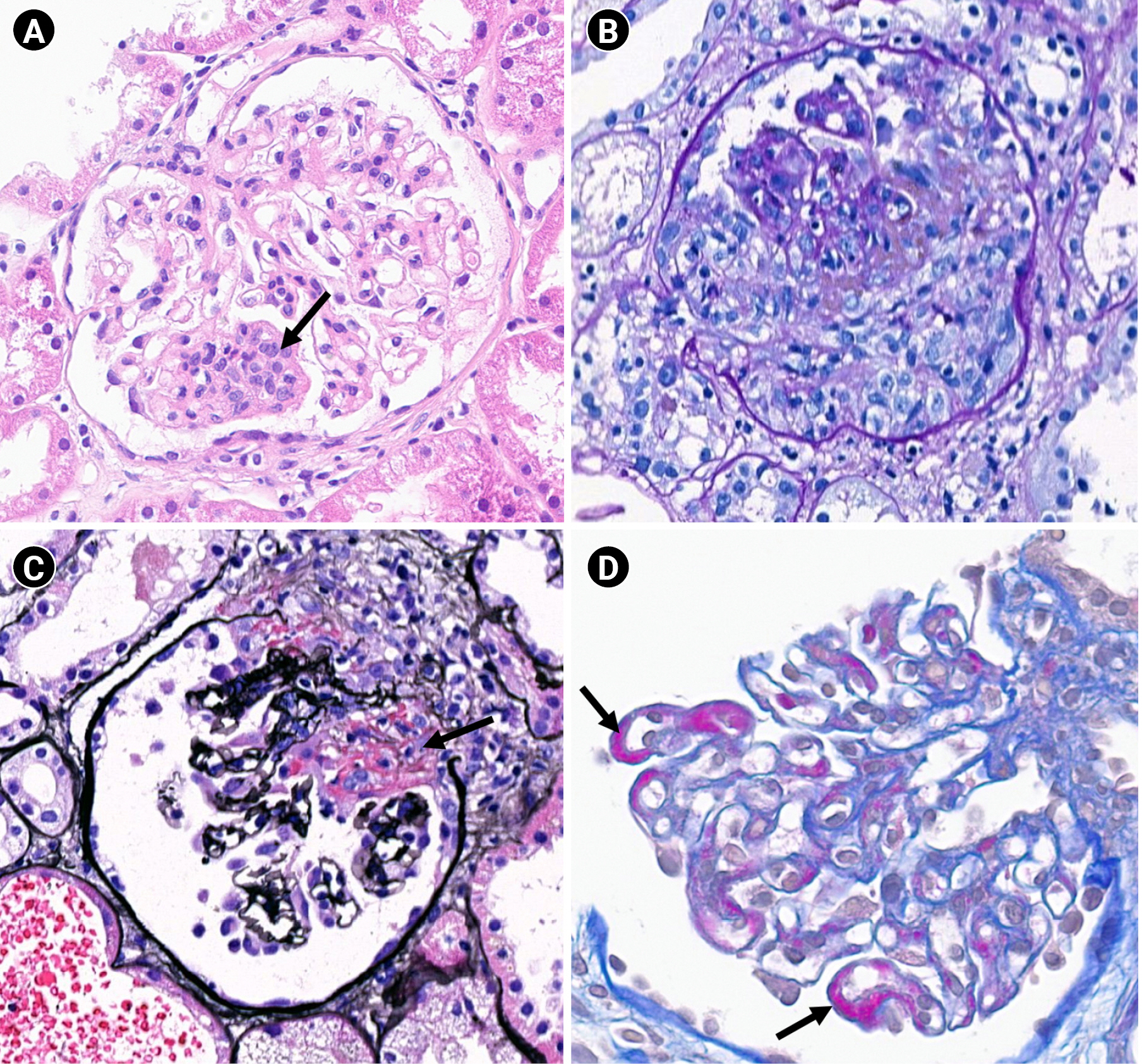
Example lesions included in the National Institutes of Health indices (×400).
(A) Endocapillary hypercellularity (arrow; hematoxylin and eosin stain). (B) Cellular crescent (periodic acid-Schiff stain). (C) Fibrinoid necrosis (arrow; periodic acid-methenamine silver stain). (D) Hyaline deposits (wire-loop lesions, arrows; acid fuchsin orange G stain).
Clinical significance of the National Institutes of Health indices
In the original report by Austin et al. [6], the NIH indices had a predictive value for identifying patients at high risk of kidney failure. Patients with an AI of 11 or more and a CI of 3 or more constituted the high-risk group [6]. When focusing on cases of diffuse proliferative lupus nephritis, AI, CI, and individual components of the CI had predictive value, with high-risk patients scoring 12 or more points in the AI and 4 or more points in the CI [7]. The predictive values of both the AI and CI were consistent in patients with severe lupus nephritis who had been treated with either cyclophosphamide or methylprednisolone [19].
We will now review a number of studies assessing the predictive powers of AI and CI. The studies are arranged according to the similarity of conclusions and not chronologically. Magil et al. [10] used the same parameters and scoring methods as reported by Austin et al. [6] in a study of diffuse proliferative lupus nephritis and reported that AI, as well as other clinical and histologic features, predicted outcomes. Interestingly, weighting the scores of cellular crescents and fibrinoid necrosis/karyorrhexis did not affect the significance of the AI [10]. Esdaile et al. [11–14] also adopted the NIH indices in their studies and demonstrated the predictive values of both the AI and CI in renal outcomes. Arce-Salinas et al. [20] reported that patients with diffuse proliferative lupus nephritis with an AI score higher than 9 had significantly higher risk of developing chronic renal failure than patients with an AI score less than or equal to 9; Yokoyama et al. [21] reported that AI was the most significant risk factor for death and/or end-stage kidney disease after initial kidney biopsies. However, Appel et al. [22] reported that the AI and CI did not significantly predict kidney outcomes, and a substantial proportion of mesangial lesions accounted for these discrepancies. Schwartz et al. [23] also reported that AI and CI did not significantly predict kidney failure or death in patients treated for severe lupus nephritis. Levey et al. [24] conducted a multicenter, randomized controlled study and did not find significant differences in AI and CI between groups of patients with and without kidney failure who had been treated for severe lupus nephritis. Wu et al. [25] found that both the AI and CI were significant predictive factors for kidney outcomes in a univariate Cox hazard analysis, and the CI remained significantly predictive in multivariate analysis. Hsieh et al. [26] performed a cohort study and showed that patients with a CI greater than 3 were more likely to progress to kidney failure, whereas the AI was not predictive of kidney survival.
However, Schwartz et al. [27] also demonstrated the irreproducibility of the AI and CI among four pathologists, showing a reliability coefficient of 0.48 for AI and 0.57 for the CI. These authors attributed the low reliability coefficient to interpretative differences. Dasari et al. [28] analyzed pathologist concordance using the ISN/RPS classification and the AI and CI of patients with lupus nephritis after reviewing six studies with at least four pathologists involved in each study. These authors concluded that the AI and CI exhibit poor interobserver agreement and are therefore limited for clinical use.
Since its inception, studies assessing the prognostic importance of the AI have concluded it has low utility, and most data collected over time indicate that the AI is not associated with kidney outcome [22–26]. The reason for this shift in utility may be due to responsiveness of certain active lesions to immunosuppressants. For example, patients with high AI scores may be more likely to receive more aggressive immunosuppressive therapy, and when these therapies are effective, AI score would not be associated with worse outcome. The Oxford study on immunoglobulin A (IgA) nephropathy previously suggested that endocapillary hypercellularity is a lesion that is more responsive to immunosuppressants due to a lack of association with kidney failure in patients who received immunosuppression [29]. Further studies showed that crescents in IgA nephropathy were not associated with worse outcome if present in <25% of glomeruli when the patient was treated with immunosuppression [30]. Similarly, some researchers suggested that the endocapillary hypercellularity of lupus nephritis may be reversible in patients using immunosuppressants, given the lack of the lesion’s association with a decline in kidney function [31,32].
Revision of the National Institutes of Health indices and their incorporation into the International Society of Nephrology/Renal Pathology Society classification
The original ISN/RPS classification referred to the NIH indices as a possible supplement for the A, C, and A/C subclasses of lupus nephritis classes III and IV [1,2] because these subclasses do not describe the extent of active and chronic lesions [3]. In the 2018 proposed revision, the NIH indices were modified, and it was suggested they be incorporated into the ISN/RPS classification, even replacing the A, C, and A/C subclasses. The proposed modification included separating karyorrhexis from fibrinoid necrosis and combining it with neutrophil infiltration (leukocyte exudation in the original NIH indices) and including fibrocellular crescents as well as cellular crescents in AI and to provide clearer definitions of these lesions (Table 1) [4]. Importantly, this incorporation does not mean that the scientific basis of the NIH indices has been confirmed. The NIH indices were not originally established using an evidence-based approach, and clinical validation of these indices has not been thoroughly investigated, as mentioned above. Therefore, the authors of the proposed revision of the ISN/RPS classification proposed that the validity of the NIH indices should be confirmed using an evidence-based approach in a phase 2 modification of classification of kidney lesions in SLE [4].
Validation studies of the modified National Institutes of Health indices
Phase 2 analysis to assess the validity of the NIH indices has not yet been done; however, there have been several validation studies of the modified NIH indices. Tao et al. [33] performed a retrospective validation study of the revised ISN/RPS classification system in a Chinese cohort at a single institution. They evaluated the diagnostic reproducibility of each histologic parameter of the revised ISN/RPS classification, the correlation of histologic parameters and clinical/laboratory features at the time of biopsy, and the correlation between histologic parameters and long-term outcomes. The authors observed that the modified CI, compared with the original CI, had a better correlation with composite outcomes, including death, progression to end-stage kidney disease, and a 30% reduction in the estimated glomerular filtration rate (eGFR). The original CI did not correlate with outcomes. The modified AI did not show significant differences in correlations versus the original AI. The authors also showed that the diagnostic reproducibility of assessment of cellular crescents was poor but was markedly increased when cellular and fibrocellular crescents were considered together [33]. We think it is possible that the clear distinction between the types of crescents was the reason for the increased correlation of the modified CI with outcomes. Umeda et al. [34] also performed a retrospective comparison of the original and revised ISN/RPS classifications, including the NIH indices, at a single Japanese institution. They compared the modified NIH indices with the A, C, and A/C subclasses of the original ISN/RPS classification in terms of their correlation with a 30% decline in eGFR. Their analysis indicated that the revised ISN/RPS classification and the modified NIH indices were superior to the original versions. The modified CI (but not the modified AI) correlated with kidney outcomes, while subclass type did not. The incorporation of the modified NIH indices into the revised ISN/RPS classification was evidence of progress, as the authors discussed, considering that tubulointerstitial lesions were included in the classification and that modified NIH indices provided information on the quantity of histologic parameters, even in cases of lupus nephritis classes I, II, and V. It is also worth noting that the authors used cut-off scores of 8 (≤8 vs. ≥9) for the modified AI and 4 (≤4 vs. ≥5) for the modified CI to compare kidney outcomes. Another retrospective validation study focusing on the clinical usefulness of the modified NIH indices was conducted by Nakagawa et al. [35] in a cohort at a single Japanese center. The authors grouped patients with low, moderate, and high AI and CI scores (low: 0–5, moderate: 6–11, and high: 12–24 for the AI; and low: 0–2, moderate: 3–5, and high: 6–12 for the CI). Using a multivariable analysis and adjusting for age and serum creatinine levels, moderate and high modified CI scores were significantly correlated with composite endpoints of end-stage kidney disease or all-cause death. The modified AI was not a risk factor for outcomes in multivariable analysis. A study from Thailand demonstrated that revised CI was one of the most significant predictors of clinical remission of so-called “proliferative” lupus nephritis after induction therapy, although the study was performed in a small group of patients [36]. Navarro et al. [37] analyzed the predictability of Austin’s morphological indices in a study at a single Portuguese center. Although the definition of indices differed slightly from that of the modified NIH indices, increased CI still correlated with worse kidney function and proteinuria at the end of follow-up. AI correlated with laboratory parameters reflective of immunological activity (C3, C4, and anti–double-stranded DNA) at presentation. Another retrospective multicenter cohort study comparing the predictability of the A/C subclass and the modified NIH indices (AI and CI) was performed by Hachiya et al. [38]. In this study, a higher CI score (not A/C subclass or AI) was associated with both decline in kidney function (as defined by a 1.5-fold increase in serum creatinine level) and complete remission by Cox regression analysis.
Histologic indicators of disease activity other than National Institutes of Health indices
There have been attempts to invent a morphologic index that is more closely correlated with a patient’s prognosis and predictive of treatment response than the NIH indices. Of those, one is the well-known Biopsy Index proposed by Hill et al. [39]. By modifying the standard AI and CI with the addition of new indices encompassing tubulointerstitial lesions and immunofluorescent findings, Hill et al. [39] developed this index, which is a combination of four biopsy indices: the Glomerular Activity Index, the Tubulointerstitial Activity Index, the Chronic Lesions Index, and the Immunofluorescence Index. Conclusive analyses and comparisons with other histologic indices have revealed that the Biopsy Index is more significantly associated with parameters of clinical and kidney survival, both for the initial biopsy and for patients with a second protocol biopsy that was performed after 6 months of induction therapy. However, Rathi et al. [40] found no significant correlation between the Biopsy Index and parameters of clinical outcomes; however, the sample size was small and retrospectively analyzed.
Conclusion
Since they were proposed by Austin, there have been many studies on the clinical significance of the NIH indices. At first, it seemed that both the AI and CI were correlated with kidney outcomes. Over time, the CI proved to be more strongly predictive of kidney outcomes than the AI, possibly reflecting changes in therapeutic strategies for treatment of active lesions. The A/C subclass was proposed to be substituted by the modified NIH indices in the 2018 ISN/RPS revised classification of lupus nephritis with the caveat that an evidence-based approach should be followed for validation. Several validation studies have demonstrated a stronger correlation of the modified CI to kidney outcome than either the original NIH AI or CI. Given the less predictive power of the modified AI, the modified CI is expected to be recognized as the primary parameter for kidney outcomes of lupus nephritis. The next revision of the lupus nephritis classification should further clarify this issue.
Notes
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2019R1F1A1058877).
Authors’ contributions
Conceptualization: All authors
Funding acquisition: BJL
Writing–original draft: SEC
Writing–review & editing: ABF, BJL
All authors read and approved the final manuscript.

