1. Moranne O, Froissart M, Rossert J, et al. Timing of onset of CKD-related metabolic complications.
J Am Soc Nephrol 2009;20:164–171.



2. Bushinsky DA, Chabala JM, Gavrilov KL, Levi-Setti R. Effects of in vivo metabolic acidosis on midcortical bone ion composition.
Am J Physiol 1999;277:F813–F819.


3. Bellasi A, Di Micco L, Santoro D, et al. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease.
BMC Nephrol 2016;17:158.



4. Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway.
J Clin Invest 1996;97:1447–1453.



5. Yenchek R, Ix JH, Rifkin DE, et al. Association of serum bicarbonate with incident functional limitation in older adults.
Clin J Am Soc Nephrol 2014;9:2111–2116.



6. Dobre M, Gaussoin SA, Bates JT, et al. Serum bicarbonate concentration and cognitive function in hypertensive adults.
Clin J Am Soc Nephrol 2018;13:596–603.



7. Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD.
Nephrol Dial Transplant 2009;24:1232–1237.


8. Navaneethan SD, Schold JD, Arrigain S, et al. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease.
Clin J Am Soc Nephrol 2011;6:2395–2402.



9. Djamali A, Singh T, Melamed ML, et al. metabolic acidosis 1 year following kidney transplantation and subsequent cardiovascular events and mortality: an observational cohort study.
Am J Kidney Dis 2019;73:476–485.


10. Wesson DE, Pruszynski J, Cai W, Simoni J. Acid retention with reduced glomerular filtration rate increases urine biomarkers of kidney and bone injury.
Kidney Int 2017;91:914–927.


11. Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3.
J Clin Invest 1985;76:667–675.



12. Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors.
Kidney Int 2008;73:192–199.


13. Wesson DE, Jo CH, Simoni J. Angiotensin II-mediated GFR decline in subtotal nephrectomy is due to acid retention associated with reduced GFR.
Nephrol Dial Transplant 2015;30:762–770.


14. Harambat J, Kunzmann K, Azukaitis K, et al. Metabolic acidosis is common and associates with disease progression in children with chronic kidney disease.
Kidney Int 2017;92:1507–1514.


15. Dobre M, Yang W, Chen J, et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study.
Am J Kidney Dis 2013;62:670–678.



16. Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans.
Kidney Int 2011;79:356–362.


17. Menon V, Tighiouart H, Vaughn NS, et al. Serum bicarbonate and long-term outcomes in CKD.
Am J Kidney Dis 2010;56:907–914.


18. Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study.
Am J Kidney Dis 2009;54:270–277.



19. de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status.
J Am Soc Nephrol 2009;20:2075–2084.



20. Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy.
Kidney Int 2010;78:303–309.


21. Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy.
Kidney Int 2012;81:86–93.


22. Dubey AK, Sahoo J, Vairappan B, Haridasan S, Parameswaran S, Priyamvada PS. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: a randomized controlled trial.
Nephrol Dial Transplant 2020;35:121–129.


23. Di Iorio BR, Bellasi A, Raphael KL, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study.
J Nephrol 2019;32:989–1001.



24. Navaneethan SD, Shao J, Buysse J, Bushinsky DA. Effects of treatment of metabolic acidosis in CKD: a systematic review and meta-analysis.
Clin J Am Soc Nephrol 2019;14:1011–1020.


25. National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease.
Am J Kidney Dis 2003;42(4 Suppl 3):S1–S201.

26. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150.
27. Rose BD, Post TW, Stokes J. Clinical physiology of acid base and electrolyte disorders. 6th ed. New York: McGraw-Hill; 2017.
28. Halperin ML, Goldstein MB. Fluid, electrolyte, and acid-base physiology. 5th ed. Philadelphia: WB Saunders; 2016.
29. Sakurabayashi I. Blood pH. In: Sakurabayashi I, Kumasaka K, Ito K, Miyaji Y, , Laboratory medicine encyclopedia & dictionary. Tokyo: Ishiyaku Publishers, Inc.; 2008.
30. Kraut JA, Raphael KL. Assessment of acid-base status: beyond serum bicarbonate.
Clin J Am Soc Nephrol 2021;16:1429–1431.


31. Kajimoto S, Sakaguchi Y, Asahina Y, Kaimori JY, Isaka Y. Modulation of the association of hypobicarbonatemia and incident kidney failure with replacement therapy by venous pH: a cohort study.
Am J Kidney Dis 2021;77:35–43.


32. Raphael KL, Murphy RA, Shlipak MG, et al. Bicarbonate concentration, acid-base status, and mortality in the health, aging, and body composition study.
Clin J Am Soc Nephrol 2016;11:308–316.



33. Husted FC, Nolph KD, Maher JF. NaHCO
3 and NaC1 tolerance in chronic renal failure.
J Clin Invest 1975;56:414–419.



34. Husted FC, Nolph KD. NaHCO
3 and NaCl tolerance in chronic renal failure II.
Clin Nephrol 1977;7:21–25.

35. Garofalo C, Provenzano M, Andreucci M, et al. Predictive effect of salt intake on patient and kidney survival in non-dialysis CKD: competing risk analysis in older versus younger patients under nephrology care.
Nephrol Dial Transplant 2021;36:2232–2240.

36. Kendrick J, Shah P, Andrews E, et al. Effect of treatment of metabolic acidosis on vascular endothelial function in patients with CKD: a pilot randomized cross-over study.
Clin J Am Soc Nephrol 2018;13:1463–1470.



37. Madias NE. Metabolic acidosis and CKD progression.
Clin J Am Soc Nephrol 2021;16:310–312.


38. Kim HJ, Kang E, Ryu H, et al. Metabolic acidosis is associated with pulse wave velocity in chronic kidney disease: results from the KNOW-CKD Study.
Sci Rep 2019;9:16139.



39. Duranton F, Cohen G, De Smet R, et al. Normal and pathologic concentrations of uremic toxins.
J Am Soc Nephrol 2012;23:1258–1270.



40. Wang X, Yang S, Li S, et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents.
Gut 2020;69:2131–2142.


41. Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review.
J Am Soc Nephrol 2014;25:1897–1907.



42. Gupta N, Buffa JA, Roberts AB, et al. Targeted inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease.
Arterioscler Thromb Vasc Biol 2020;40:1239–1255.



43. Wesson DE, Mathur V, Tangri N, et al. Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: a multicentre, randomised, blinded, placebo-controlled, 40-week extension.
Lancet 2019;394:396–406.


44. Bushinsky DA, Hostetter T, Klaerner G, et al. Randomized, controlled trial of TRC101 to increase serum bicarbonate in patients with CKD.
Clin J Am Soc Nephrol 2018;13:26–35.


45. Adrogué HJ, Madias NE. Veverimer: an emerging potential treatment option for managing the metabolic acidosis of CKD.
Am J Kidney Dis 2020;76:861–867.


46. Wesson DE, Mathur V, Tangri N, et al. Veverimer versus placebo in patients with metabolic acidosis associated with chronic kidney disease: a multicentre, randomised, double-blind, controlled, phase 3 trial.
Lancet 2019;393:1417–1427.


47. Asahina Y, Sakaguchi Y, Kajimoto S, et al. Association of time-updated anion gap with risk of kidney failure in advanced CKD: a cohort study.
Am J Kidney Dis 2022;79:374–382.

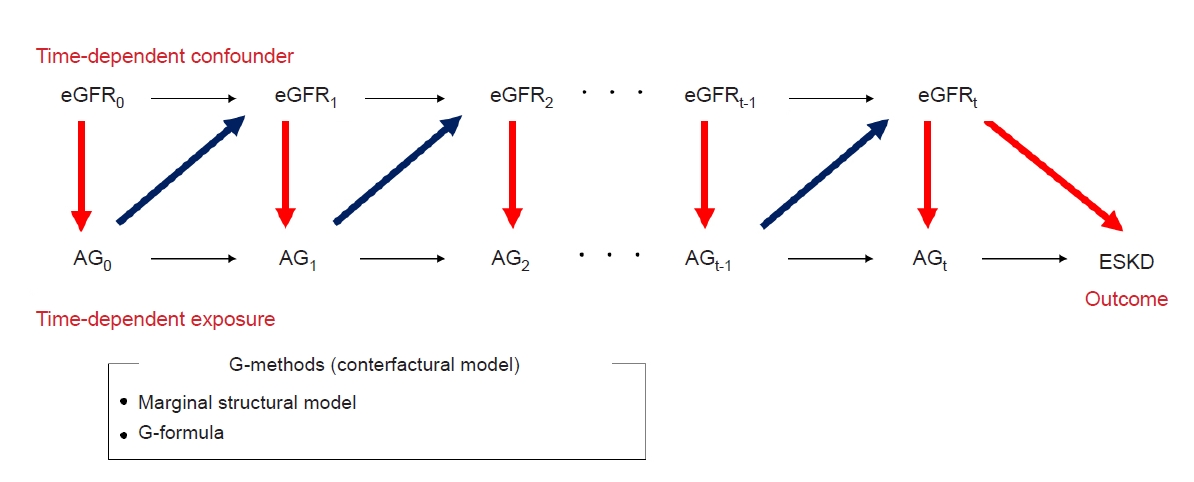
48. Mansournia MA, Etminan M, Danaei G, Kaufman JS, Collins G. Handling time varying confounding in observational research.
BMJ 2017;359:j4587.


49. Naimi AI, Cole SR, Kennedy EH. An introduction to g methods.
Int J Epidemiol 2017;46:756–762.


50. Tanemoto M. Gap acidosis except lactic acidosis develops and progresses during chronic kidney disease stage G5.
Clin Exp Nephrol 2019;23:1045–1049.


51. Banerjee T, Crews DC, Wesson DE, McCulloch CE, Johansen KL, Saydah S, et al. Elevated serum anion gap in adults with moderate chronic kidney disease increases risk for progression to end-stage renal disease.
Am J Physiol Renal Physiol 2019;316:F1244–F1253.



52. Mair RD, Sirich TL, Plummer NS, Meyer TW. Characteristics of colon-derived uremic solutes.
Clin J Am Soc Nephrol 2018;13:1398–1404.



53. Koepsell H, Endou H. The SLC22 drug transporter family.
Pflugers Arch 2004;447:666–676.


54. Nigam SK. What do drug transporters really do?
Nat Rev Drug Discov 2015;14:29–44.


55. Chen Y, Zelnick LR, Hoofnagle AN, et al. Prediction of kidney drug clearance: a comparison of tubular secretory clearance and glomerular filtration rate.
J Am Soc Nephrol 2021;32:459–468.


56. Aronov PA, Luo FJ, Plummer NS, et al. Colonic contribution to uremic solutes.
J Am Soc Nephrol 2011;22:1769–1776.



57. Poesen R, Windey K, Neven E, et al. The influence of CKD on colonic microbial metabolism.
J Am Soc Nephrol 2016;27:1389–1399.


58. Chen Y, Zelnick LR, Wang K, et al. Kidney clearance of secretory solutes is associated with progression of CKD: the CRIC study.
J Am Soc Nephrol 2020;31:817–827.



59. Takayama F, Taki K, Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis.
Am J Kidney Dis 2003;41(3 Suppl 1):S142–S145.


60. Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin.
Nephrol Dial Transplant 2010;25:219–224.


61. Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients.
Clin J Am Soc Nephrol 2014;9:1603–1610.



62. Nakabayashi I, Nakamura M, Kawakami K, et al. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study.
Nephrol Dial Transplant 2011;26:1094–1098.


63. Guida B, Germanò R, Trio R, et al. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial.
Nutr Metab Cardiovasc Dis 2014;24:1043–1049.


64. Rossi M, Johnson DW, Morrison M, et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial.
Clin J Am Soc Nephrol 2016;11:223–231.

















 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















