| Kidney Res Clin Pract > Volume 41(1); 2022 > Article |
|
Abstract
Background
Methods
Results
Notes
Funding
This study was partially supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean Ministry of Science and Information and Communications Technology (MSIT; NRF-2019M3E5D3073102) and Soonchunhyang University Research Fund.
AuthorsŌĆÖ contributions
Conceptualization: SHK, BDN
Data curation, Formal analysis: SHK, BDN, NJC, HK, SP
Funding acquisition: SHK
Investigation: BDN, HK, HN, JSJ, DCH, EYL, HWG
Methodology: SHK, BDN
Supervision: SHK, BDN, HN, JSJ, DCH, EYL, HWG
WritingŌĆōoriginal draft: NJC
WritingŌĆōreview & editing: BDN, SHK
All authors read and approved the final manuscript.
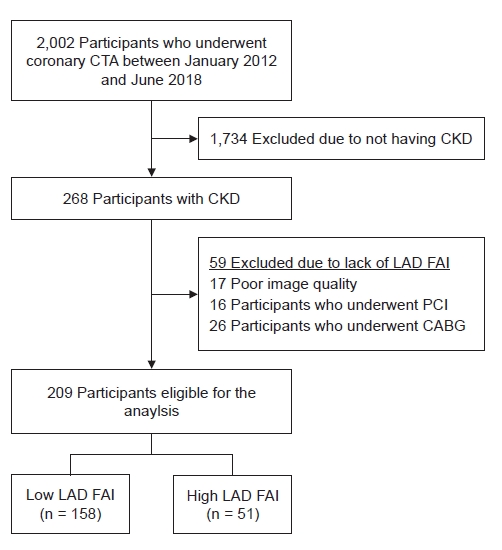
Figure┬Ā1.
Study design and flowchart.
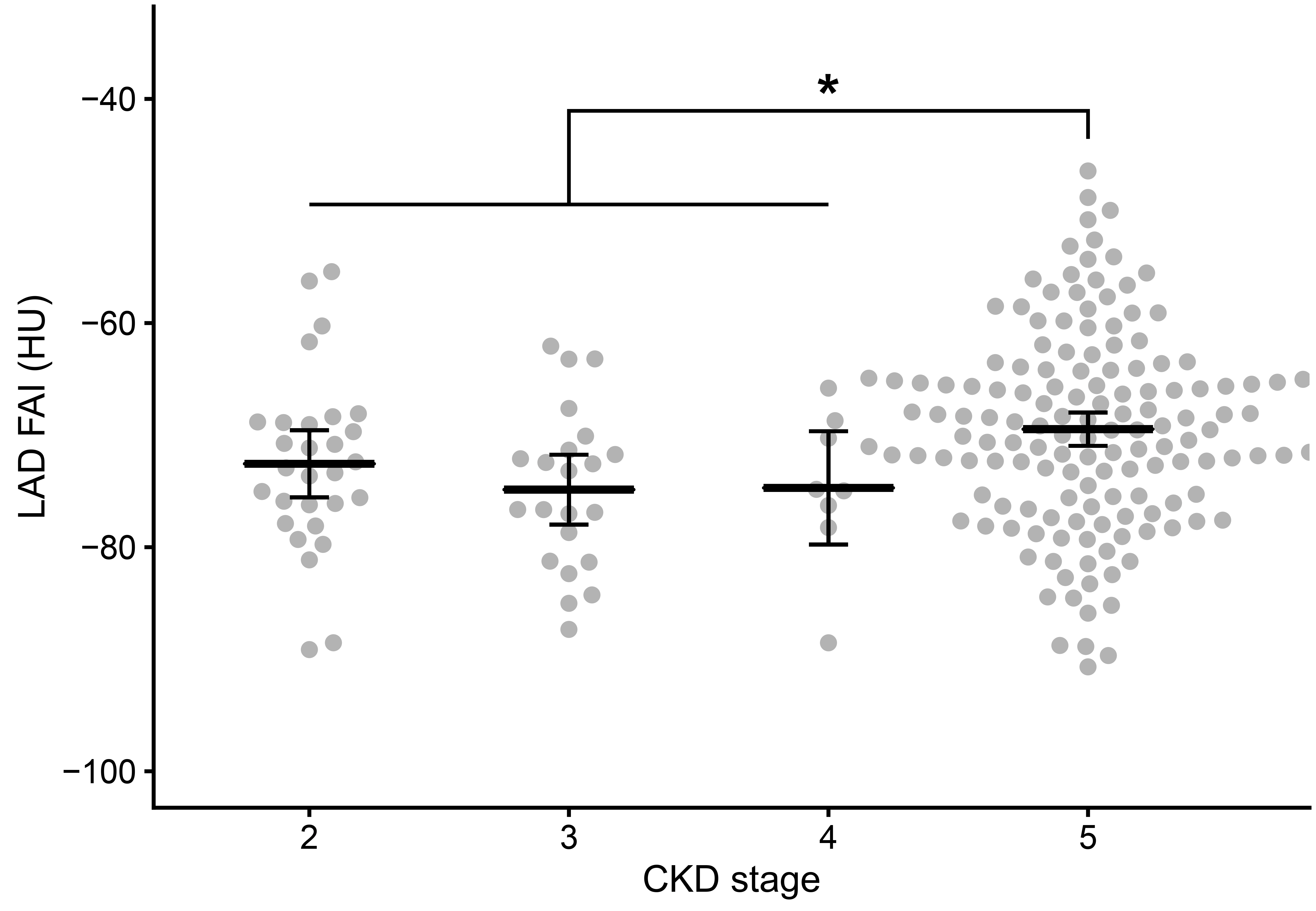
Figure┬Ā2.
LAD FAI values according to CKD stage.
Figure┬Ā3.
Comparative risks of death between the high- and low-LAD-FAI groups.

Figure┬Ā4.
Nonlinear associations between the hazard ratios of all-cause death and the LAD FAI values.

Table┬Ā1.
| Characteristic | All patients (n = 209) | Low-LAD-FAI (N = 158) | High LAD FAI (N = 51) | p-value |
|---|---|---|---|---|
| LAD FAI range (HU) | ŌłÆ99.1 to ŌłÆ50.8 | ŌłÆ99.1 to ŌłÆ65.5 | ŌłÆ65.5 to ŌłÆ50.8 | |
| Age (yr) | 63.3 ┬▒ 12.4 | 64.1 ┬▒ 12.6 | 60.9 ┬▒ 11.6 | 0.11 |
| Male sex | 100 (47.8) | 72 (45.6) | 28 (54.9) | 0.32 |
| Body mass index (kg/m2) | 23.4 ┬▒ 3.7 | 24.4 ┬▒ 3.5 | 20.6 ┬▒ 3.0 | <0.001 |
| Smoking history | 0.64 | |||
| ŌĆāNonsmoker | 135 (64.6) | 102 (64.6) | 33 (64.7) | |
| ŌĆāExsmoker | 30 (14.4) | 21 (13.3) | 9 (17.6) | |
| ŌĆāCurrent smoker | 44 (21.1) | 35 (22.2) | 9 (17.6) | |
| CKD stage | 0.04 | |||
| ŌĆā2 | 30 (14.4) | 26 (16.5) | 4 (7.8) | |
| ŌĆā3 | 24 (11.5) | 21 (13.3) | 3 (5.9) | |
| ŌĆā4 | 8 (3.8) | 8 (5.1) | 0 (0.0) | |
| ŌĆā5 | 147 (70.3) | 103 (65.2) | 44 (86.3) | |
| Dialysis statusa, yes | 147 (70.3) | 104 (65.8) | 43 (84.3) | 0.02 |
| DKD, present | 85 (40.7) | 61 (38.6) | 24 (47.1) | 0.37 |
| Hemoglobin (g/dL) | 10.9 ┬▒ 2.0 | 11.1 ┬▒ 2.2 | 10.5 ┬▒ 1.5 | 0.03 |
| Albumin (g/dL) | 4.03 ┬▒ 0.54 | 4.06 ┬▒ 0.54 | 3.92 ┬▒ 0.51 | 0.10 |
| Urea nitrogen (mg/dL) | 40.7 (20.8ŌĆō60.1) | 35.5 (20.6ŌĆō57.6) | 45.8 (27.2ŌĆō63.4) | 0.28 |
| Creatinine (mg/dL) | 6.93 (1.38ŌĆō9.75) | 6.79 (1.31ŌĆō9.78) | 7.50 (5.56ŌĆō9.70) | 0.08 |
| eGFR (mL/min/1.73 m2) | 6.18 (4.45ŌĆō45.51) | 6.50 (4.45ŌĆō50.68) | 6.00 (4.49ŌĆō9.92) | 0.04 |
| Calcium (mg/dL) | 9.00 (8.62ŌĆō9.45) | 8.98 (8.65ŌĆō9.39) | 9.10 (8.51ŌĆō9.61) | 0.82 |
| Phosphorus (mg/dL) | 4.36 ┬▒ 1.62 | 4.31 ┬▒ 1.60 | 4.52 ┬▒ 1.70 | 0.43 |
| Total cholesterol (mg/dL) | 152 ┬▒ 41 | 155 ┬▒ 41 | 143 ┬▒ 40 | 0.10 |
| Triglyceride (mg/dL) | 112 (84ŌĆō160) | 118 (88ŌĆō168) | 101 (68ŌĆō125) | 0.01 |
| Ejection fraction (%) | 61.0 (50.5ŌĆō67.0) | 62.0 (55.8ŌĆō68.0) | 58.0 (45.0ŌĆō64.5) | 0.009 |
| No. of stenotic vessels | 0.61 | |||
| ŌĆā0 | 44 (21.1) | 35 (22.2) | 9 (17.6) | |
| ŌĆā1 | 45 (21.5) | 34 (21.5) | 11 (21.6) | |
| ŌĆā2 | 45 (21.5) | 36 (22.8) | 9 (17.6) | |
| ŌĆā3 | 75 (35.9) | 53 (33.5) | 22 (43.1) | |
| High-risk plaque, present | 5 (2.4) | 4 (2.5) | 1 (2.0) | >0.99 |
| Maximum degree of stenosis | 0.17 | |||
| ŌĆāNo | 44 (21.1) | 35 (22.2) | 9 (17.6) | |
| ŌĆāMinimal | 23 (11.0) | 21 (13.3) | 2 (3.9) | |
| ŌĆāMild | 60 (28.7) | 41 (25.9) | 19 (37.3) | |
| ŌĆāModerate | 51 (24.4) | 40 (25.3) | 11 (21.6) | |
| ŌĆāSevere | 31 (14.8) | 21 (13.3) | 10 (19.6) |
Data are presented as range, mean ┬▒ standard deviation, number (%), or median (interquartile range). Patients were categorized by the optimum LAD FAI cutoff (ŌłÆ65.5 HU).
BMI, body mass index; CKD, chronic kidney disease; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; FAI, fat attenuation index; HU, Hounsfield unit; LAD, left anterior descending artery.
Table┬Ā2.
The ╬▓ coefficients, standard errors (SEs), and p-values were calculated using simple and multiple linear regression analyses. Multiple linear regression analyses included the variables with p-values less than 0.05 in the simple linear regression (age, body mass index, dialysis status, hemoglobin, serum albumin, total cholesterol, and triglyceride). DKD, diabetic kidney disease.
Table┬Ā3.
| Model |
All CKD patients |
Patients on dialysis |
|||
|---|---|---|---|---|---|
| Low LAD FAI | High LAD FAI | p-value | High LAD FAI | p-value | |
| 1a | 1 (reference) | 2.08 (1.12ŌĆō3.85) | 0.02 | 1.96 (1.00ŌĆō3.87) | 0.05 |
| 2b | 1 (reference) | 1.81 (0.89ŌĆō3.71) | 0.10 | 2.89 (1.19ŌĆō7.05) | 0.02 |
| 3c | 1 (reference) | 1.81 (0.88ŌĆō3.73) | 0.12 | 3.01 (1.19ŌĆō7.61) | 0.02 |
Data are presented as the hazard ratio (95% confidence interval) attained by multivariable Cox proportional hazard models. The LAD FAI values were categorized into low and high-LAD-FAI groups according to the optimum cutoff level (ŌłÆ65.5 Hounsfield unit).
CKD, chronic kidney disease; FAI, fat attenuation index; LAD, left anterior descending artery.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Nam-Jun Cho

https://orcid.org/0000-0002-9053-0499Bo Da Nam

https://orcid.org/0000-0001-7822-6104Samel Park

https://orcid.org/0000-0002-5717-0743Hyoungnae Kim

https://orcid.org/0000-0002-5359-0214Hyunjin Noh

https://orcid.org/0000-0002-1904-1684Jin Seok Jeon

https://orcid.org/0000-0003-2421-2289Dong Cheol Han

https://orcid.org/0000-0002-8835-8642Eun Young Lee

https://orcid.org/0000-0002-4513-9888Hyo-Wook Gil

https://orcid.org/0000-0003-2550-2739Soon Hyo Kwon

https://orcid.org/0000-0002-4114-4196 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















