| Kidney Res Clin Pract > Volume 38(1); 2019 > Article |
|
Abstract
Background
The prevalence of acute kidney injury (AKI) in elderly patients has grown considerably. Age-associated changes in the immune system can be one of the critical factors determining AKI outcomes. This study aimed to investigate the role of senescence of bone marrow (BM)-derived cells in the development of AKI, focusing on the immune response.
Methods
Female 7-week-old C57BL/6 mice were irradiated and treated with BM cells from either 48-week-old or 8-week-old male mice. Ischemia-reperfusion injury (IRI) was induced, and their functional deterioration, histological tubular damage, and inflammatory responses were compared. For the in-vitro study, lipopolysaccharide (LPS)-stimulated cytokine production by BM cells from old and young mice were examined.
Results
At 24 hours after IRI, there was no significant difference in the number of circulating immune cells between the mice transplanted with old or young BM cells. However, the mice with old BM cells showed less functional deterioration and histological tubular injury than those with young BM cells. Moreover, macrophage infiltration and renal cytokine interleukin (IL)-12 levels were lower in the mice with old BM cells at 24 hours post-IRI. Consistently, the in vitro study showed that LPS-induced production of cytokines interferon-γ, monocyte chemoattractant protein-1, and IL-10 was attenuated in cultured old BM cells, suggesting that age-related functional changes in these cells may lead to reduced inflammation in IRI.
In 2015, the number of people aged 65 and older in Korea was 6.5 million, or 12.8% of the population. By 2060, 41% of the population in Korea is projected to be aged 65 years and older according to the Korean National Statistical Office [1].
Along with the increase in the elderly population, the incidence of acute kidney injury (AKI) has been increasing annually, with an even greater increase among subjects older than 65 years. AKI is related to high mortality during hospitalization [2–4], and insufficient recovery results in decreased renal function and progression to chronic kidney disease (CKD) [5,6]. Age older than 65 years is a major risk factor for impaired renal recovery after AKI [6]. Therefore, a better understanding of AKI in the elderly population is necessary to improve disease outcomes in these patients.
In elderly patients with AKI, comorbidities such as diabetes mellitus, hypertension, and CKD are frequently observed [7]. The aged kidney is characterized by various structural and functional changes [8], all of which can eventually affect the outcome of AKI.
The inflammatory response plays a critical role in the pathophysiology of AKI and in progression to CKD [9,10]. Since various inflammatory cells are involved not only in disease development, but also in recovery from AKI, aging-related changes in these cells may have a significant impact on disease outcome.
Aging affects the development and function of immune cells, with reduced immune responses to pathogens and vaccines in the elderly [11]. A number of studies have evaluated the impact of immunosenescence on inflammatory diseases and have shown that aged immune cells make elderly people more vulnerable to infections. These age-related changes are associated with a reduced capacity to control systemic inflammation, resulting in an increased risk of chronic inflammation (inflammaging). Chronic inflammation induced by immunosenescence has been suggested as one of the mechanisms underlying the pathogenesis of various age-related chronic diseases such as type 2 diabetes, cardiovascular diseases, Alzheimer’s disease, and osteoporosis [11]. However, it is not easy to independently investigate whether immunosenescence affects the outcomes of inflammatory diseases apart from organ aging. In a recent large-scale study of transplant patients, an interesting result was reported in which young recipient age, regardless of the age of the kidney donor, was a robust risk factor of acute rejection after transplantation. This result suggests that, independent of other aging-related phenomena, young immune cells may have a negative impact on development of inflammatory renal disease [12].
Considering the strong association between AKI and inflammatory cells, aging of bone marrow (BM) cells might independently affect the outcome of AKI. However, little is known about this relationship. To selectively investigate the role of senescent BM-derived cells in development of AKI, chimeric young mice with aged or young BM cells were created, and the outcomes of AKI induced by ischemia-reperfusion injury (IRI) were compared.
The aim of this study was to selectively explore the impact of senescent BM cells on the outcome of experimentally induced AKI. To this end, we created a mouse model with old-young bone marrow transplantation (BMT). In brief, young (7-week-old), female mice were irradiated to remove peripheral blood cells and subsequently transfused with BM cells derived from either old (48-week-old) or young (8-week-old) male mice, resulting in old-to-young (O-Y) or young-to-young (Y-Y) BMT, respectively. After IRI, histological and biochemical examinations were carried out in both groups of chimeric mice to assess the extent of renal damage and the degree of inflammation.
Six- to eight-week-old male and female C57BL/6 mice (weight, 20–25 g) were purchased from Orient (Seongnam, Korea). All experimental protocols were approved by the Animal Care Committee of Korea University (KUIACUC-2014-128) and followed the NIH guidelines “Principles of Laboratory Animal Care.” For IRI induction, mice were anesthetized by intraperitoneal injection of 15 mg/kg of ketamine and 2.5 mg/kg of xylazine and subjected to bilateral renal pedicle clamping for 40 minutes. During the IRI, a warm pad was used to maintain constant mouse body temperature (37°C). Thereafter, the clamp was released, and reperfusion was observed for 1 minute. In this experiment, female mice transplanted with BM cells of male mice were used, and ischemia was maintained for a longer time because the female mice were more resistant to IRI compared to the male mice.
BM cells were obtained from femurs of mice at 48 and 8 weeks of age, and BMT was performed by intravenous injection of 1 × 107 BM cells into irradiated 7-week-old, female mice. To confirm successful BMT, the sex-determining region of the Y chromosome (Sry) was amplified by subjecting a peripheral blood sample to polymerase chain reaction (PCR), and the presence of a 402 bp band (Sry) after electrophoresis in 3% agarose indicated successful BMT.
At 24 hours post-IRI, serum creatinine was measured using a 070 Hitachi analyzer (Hitachi, Tokyo, Japan). Cytokines and chemokines in the renal tissues or in the supernatant of BM cell cultures were quantified using a cytometric bead array (CBA) mouse inflammation kit (BD GmbH, Heidelberg, Germany). Each kit was used according to the manufacturer’s protocol to simultaneously detect mouse interleukin (IL) 12p70, tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), monocyte chemoattractant protein 1 (MCP-1), IL-10, and IL-6.
Tubular injury was assessed in periodic acid-Schiff-stained kidney sections. For immunohistochemical staining, we used rat anti-mouse F4/80 (AbD Serotec, Kidlington, UK), Ly6G (Gr-1; BD Biosciences, San Jose, CA, USA), and transforming growth factor β1 (TGF β1;Abcam plc., Cambridge, MA, USA) antibodies. A total of 8 to 10 high power fields (HPFs) were analyzed, and the mean number of positive cells was compared between the groups.
Flow cytometry analysis of splenocytes was performed. We purchased CD4-APC, CD8-PE, F4/80-APC, and GR1-FITC antibodies from BD Biosciences or eBioscience (San Diego, CA, USA).
Real-time RT-PCR was performed using the iCycler IQ Real Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to detect inducible nitric oxide synthase (iNOS), arginase-1 and transforming growth factor beta (TGF-β) expression levels in the kidney. The reference gene (RT2 PCR Primer Set; Applied Biosystems, Foster City, CA, USA) was 18s.
Reconstitution of female mice with male BM was confirmed by detection of the Y chromosome in peripheral blood (Fig. 1A). Two weeks after BMT, we examined the number of circulating immune cells by FACS.
At 24 hours post-IRI, there were no significant differences in the proportions of splenic CD4+ T cells, Gr-1+ neutrophils, and F4/80+ macrophages between O-Y and Y-Y BMT (Fig. 1B).
At 24 hours post-IRI, serum creatinine and tubular injury score were compared between O-Y BMT and Y-Y BMT mice. The O-Y BMT mice showed significantly less functional deterioration and a lower tubular injury score than the Y-Y BMT mice. These results suggest that old BM cells had less impact on renal injury after IRI compared to young BM cells (Fig. 2). To address the effects of old and young BM cells on renal inflammation, tissue infiltration by inflammatory cells was evaluated. The number of infiltrating F4/80+ macrophages was significantly lower in O-Y BMT mice, whereas Gr-1+ neutrophil infiltration was comparable in the two groups (Fig. 3A, B). Furthermore, we examined the mRNA expression of M1 (pro-inflammatory) and M2 (anti-inflammatory) markers at 24 hours post-IRI. Although a lower expression level of the M2 marker arginase-1 was observed in O-Y BMT, this difference was not statistically significant (Fig. 3C). In addition, the expression of TGF-β mRNA, an important cytokine in the repair phase, was significantly lower in old BMT mice (Fig. 3D).
Next, renal inflammatory cytokines and chemokines were analyzed by a CBA. The concentration of IL-12 in the kidneys of O-Y BMT mice was significantly lower than that of Y-Y BMT mice at 24 hours post-IRI, whereas the levels of MCP-1 and IL-6 were comparable in the two groups (Fig. 3E). These results suggest that less pronounced macrophage infiltration and lower levels of proinflammatory cytokines may contribute to the milder post-IRI renal injury observed in O-Y BMT mice.
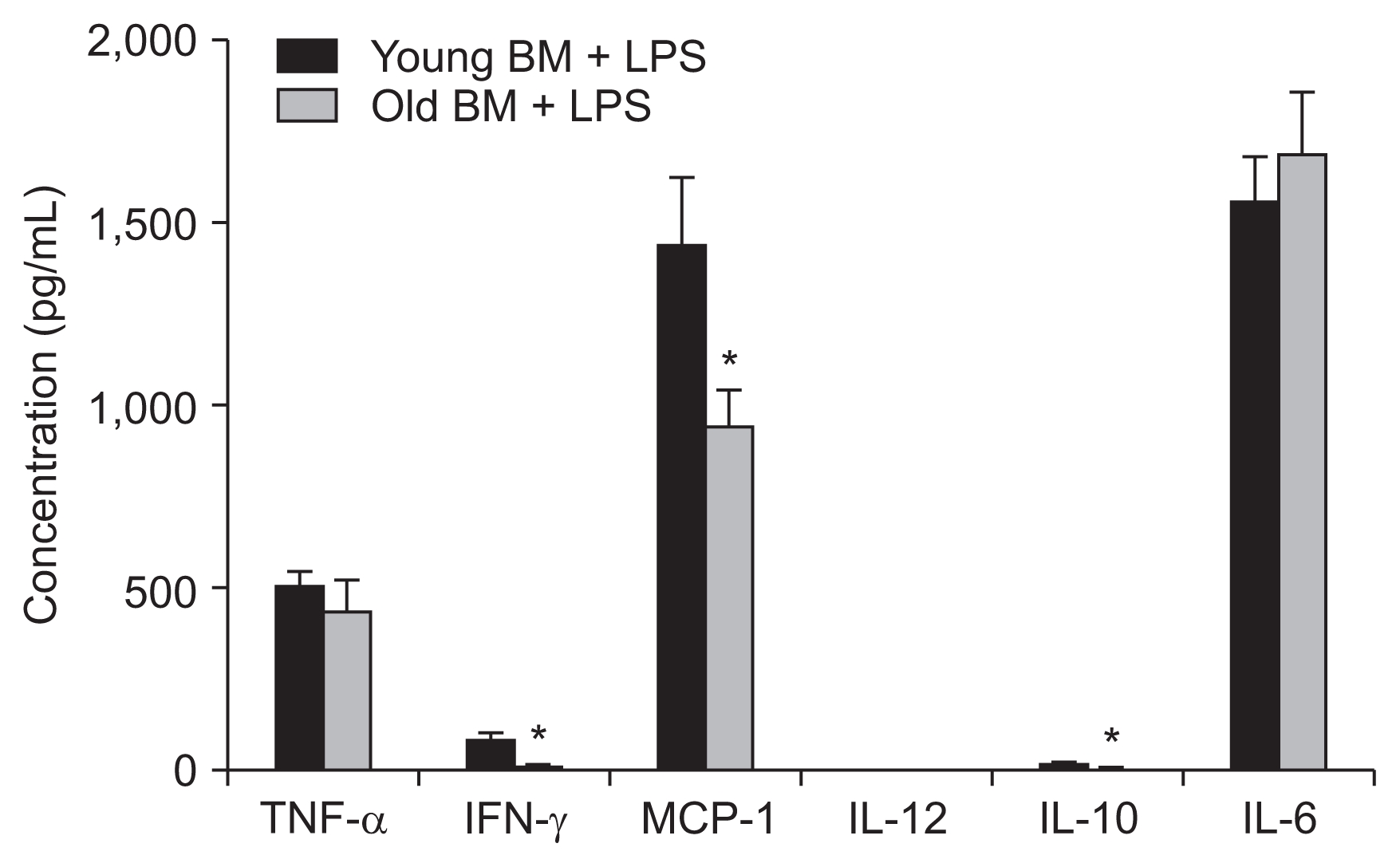
To functionally compare old and young BM cells, BM cell cultures isolated from old and young mice were exposed to LPS stimulation, and cytokine production was compared. We observed that, although the production of TNF-α and IL-6 was comparable, those of IFN-γ, MCP-1, and IL-10 were significantly decreased in old BM cells compared to young BM cells. This result suggests that a reduced response to inflammatory stimuli may contribute to the less severe inflammation, milder renal injury, and reduced post-IRI functional deteriorations observed in the O-Y BMT mice compared to the Y-Y BMT mice (Fig. 4).
Age-related disease rates are rapidly increasing with the aging of the population. Thus, conditions affecting elderly patients are a major issue in the biomedical field. One example of such conditions is AKI, the incidence of which increases with age and results in severe morbidity and high mortality among elderly patients [13,14]. Functional and structural changes of aged kidneys, as well as various comorbidities, could contribute to AKI.
Recent studies have shown that inflammation plays an important pathophysiological role in AKI [9,10]. Danger signals after IRI are known to trigger innate and adaptive immune responses in the kidney. It has been shown that inflammatory cells such as neutrophils, macrophages (M1), resident dendritic cells, natural killer T cells, and CD4 T cells are involved in AKI. Therefore, it is reasonable to think that senescence of these cells may alter the inflammatory process, possibly contributing to the different outcomes of AKI in elderly patients.
However, it is difficult to selectively study the impact of immunosenescence in human patients because confounding factors such as comorbidities, as well as age-related structural and functional changes in kidneys, cannot be controlled. Therefore, in this study, chimeric mice were created to selectively evaluate the contribution of senescent immune cells to AKI in the absence of other age-related alterations.
First, there was no difference in the population of immune cells between Y-Y and O-Y BMT mice after IRI. However, O-Y BMT mice showed less functional deterioration and histological damage after IRI compared to Y-Y BMT mice. These characteristics were accompanied, in O-Y BMT mice, by reduced infiltration of F4/80+ macrophages and a lower level of proinflammatory cytokines. In addition, in vitro LPS stimulation resulted in reduced cytokine production by old BM cells, especially IFN-γ, MCP-1, and IL-10.
Macrophages play a complex role in both IRI-induced inflammation and the subsequent repair process. Macrophages contribute to initiation of early renal injury and to development of renal fibrosis [15,16]. Recently, it was shown that the switch from a proinflammatory (M1) to an anti-inflammatory (M2) macrophage phenotype promotes the repair process in kidneys [17,18]. Considering the important role of macrophages in the acute phase of AKI, the reduced macrophage infiltration occurring in BMT mice with old BM cells could have contributed to the milder histological and functional deterioration observed in these mice after injury. In addition, aging-related functional changes in macrophages may have affected renal injury. Our data showing that IL-12 level decreased in altered macrophages, and that LPS stimulation impaired cytokine synthesis support this hypothesis.
Consistent with our data, a previous study demonstrated that decreased functionality of aged macrophages decreases mouse response to bacterial polysaccharides [19]. In that study, old macrophages displayed lower toll-like receptor 4 and CD14 expression, leading to unbalanced cytokine production in response to LPS. Thus, the reduced cytokine production we observed upon LPS stimulation of cultured senescent BM cells might be associated with the decreased inflammation following LPS. However, since immune cell activation by IRI involves damage-associated molecular patterns that are different from that of LPS-induced activation, our in vitro results have limitations in explaining macrophage activation in IRI. Another example of reported aging-related changes in monocytic or macrophagic cells is the diminished IFN-γ-dependent MHC class II transcription described in aged macrophages [20]. Thus, changes in receptor expression and macrophage responsiveness are likely to play a significant role in the reduced renal inflammation we observed in mice transplanted with aged BM cells.
Macrophages are also involved in resolution of inflammation by clearing apoptotic cells and cellular debris. This process results in transition of macrophages to an anti-inflammatory M2 phenotype. Therefore, the reduced responsiveness of senescent macrophages might affect macrophage polarization from M1 to M2 and recovery after AKI. In our study, relative expression of M2 macrophages was slightly reduced at day 1 post-IRI, suggesting aging-related impairment of macrophage polarization. However, our experiments only focused on the acute phase and did not evaluate macrophage polarization and its effect on the recovery phase. Therefore, the effect of aging on macrophage phenotypes during post-AKI recovery should be clarified in future studies.
Recent studies in old mice have shown that severity of AKI is generally similar between old and young mice [21,22]. Jang et al [21] demonstrated that aging has minor effects on initial ischemic AKI, despite the changing intrarenal immunologic micromilieu in mice. We also observed no significant differences in the degree of tubular injury and renal function at post-IRI day 1 when IRI was induced in old and young mice without BMT (data not shown). A possible explanation for this apparent discordance is that, in old animals, the structural and functional changes accumulating in the aged kidney may counteract the potentially favorable impact exerted by senescent inflammatory cells on the severity of AKI, ultimately resulting in negligible differences in disease outcome.
Most recently, Liu et al [23] have reported that, in the presence of a young systemic milieu, the effects of renal IRI are less severe in elderly mice. In the study, young-old parabiosis, consisting of aged mice with a youthful (12-week-old) systemic milieu, showed less renal histological post-ischemic injury and better renal function than the old-old parabiosis mice. These findings conflict with our evidence of reduced AKI severity in young mice with old BM cells. However, the donors and recipients were opposite to our study, and the impact of young peripheral blood on disease outcome was evaluated in old mice, i.e., in the presence of comorbidities as well as structural and functional, age-related changes. Moreover, parabiosis was not preceded by host cell depletion. These differences in experimental design might account for the apparent inconsistency in results.
In conclusion, aged BM cells showed a significant favorable effect in the acute phase of AKI, partially due to decreased inflammatory response. In clinical situations, many confounding factors such as diabetes, hypertension, cancer, and drug exposure as well as cell senescence may have an impact on altered AKI susceptibility and outcomes in the elderly population. Therefore, further studies on aging-related changes in various other cells and organs involved in AKI and in BM cells are needed to define the pathological mechanisms underlying AKI in the elderly and to develop appropriate therapeutic strategies.
Acknowledgments
This study was supported by a Korea University grant (project no. K1816581). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figure 1
No significant changes in immune cell population after BMT
(A) Reconstitution of chimeric female (F) mice with male (M) BM cells was confirmed by detecting the Y chromosome in peripheral blood. (B) The proportion of immune cells in the spleen was not different between old-to-young BMT and young-to-young BMT at 24 hours post-IRI. n = 5 per group.
BM, bone marrow; BMT, BM transplantation; IRI, ischemia-reperfusion injury; PCR, polymerase chain reaction.
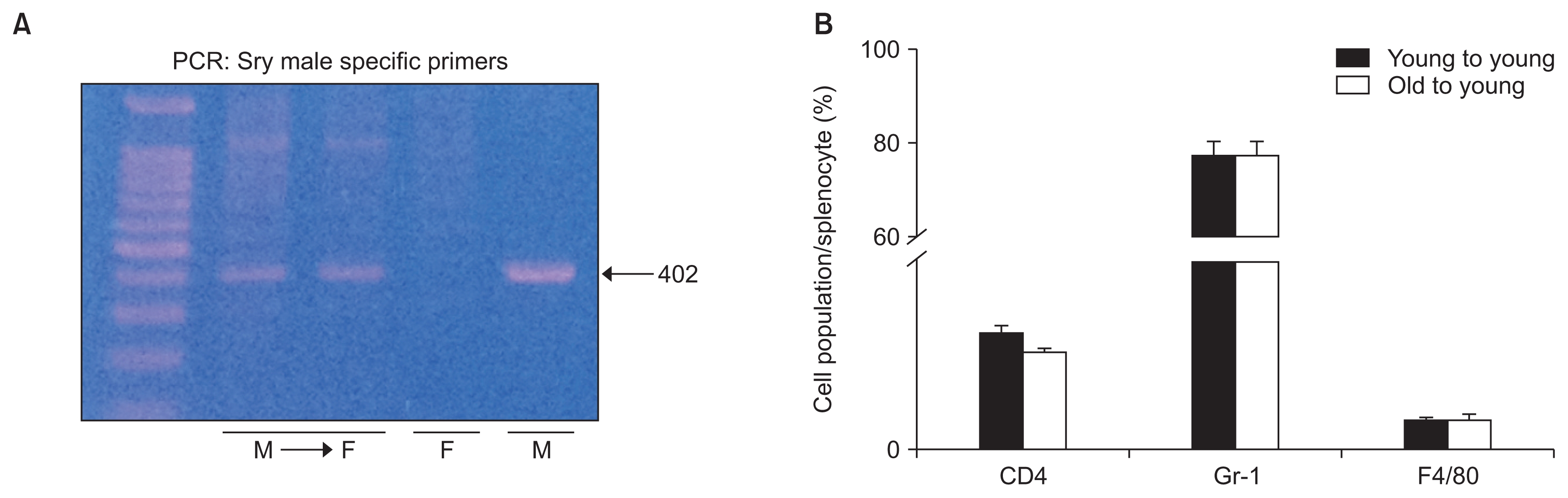
Figure 2
Renal ischemia-reperfusion injury after old or young bone marrow cell transplantation
Old-to-young (O-Y) BMT mice showed significantly less functional deterioration at 24 hours post-IRI compared to young-to-young (Y-Y) BMT mice. The tubular injury score was also significantly lower in O-Y BMT mice than in Y-Y BMT mice. Periodic acid–Schiff stain, ×100. n = 4–5 per group, *P < 0.05 compared to Y-Y BMT. Cr, creatinine; BMT, bone marrow transplantation; IRI, ischemia-reperfusion injury.

Figure 3
Post-IRI inflammation after BMT
(A) The number of renal F4/80+ macrophages was significantly lower in old-to-young (O-Y) BMT mice than in young-to-young (Y-Y) BMT mice at 24 hours post-IRI. Magnification, ×100. (B) The two groups had similar numbers of renal Gr-1+ neutrophils. Magnification, ×100. (C) The expression of arginase-1 relative to iNOS was lower in the O-Y BMT mice, although the difference was not statistically significant. (D) TGF-β mRNA expression was significantly lower in O-Y BMT mice than in Y-Y BMT mice. (E) The concentration of IL-12 in the kidneys of O-Y BMT mice was significantly lower than that of Y-Y BMT mice at 24 hours post-IRI, but there was no significant difference between the two groups in MCP-1 and IL-6. n = 4–5 per group, *P < 0.05 compared to Y-Y BMT.
BMT, bone marrow transplantation; HPF, high power field; IFN-γ, interferon-γ; IL, interleukin; iNOS, inducible nitric oxide synthase; IRI, ischemia-reperfusion injury; MCP, monocyte chemoattractant protein; mRNA, messenger RNA; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α.
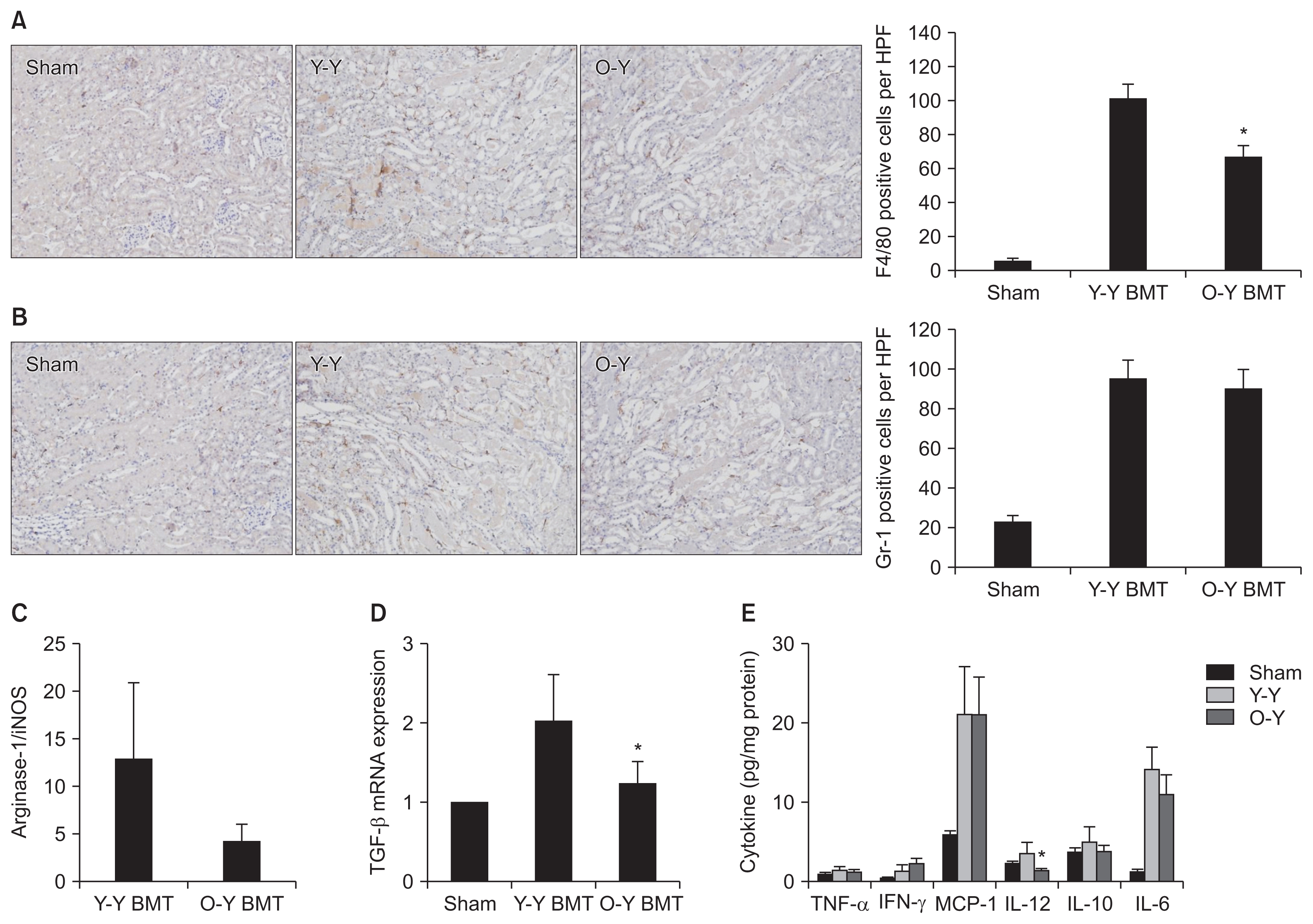
Figure 4
Cytokine/chemokine production by old or young bone marrow (BM) cells upon lipopolysaccharide (LPS) stimulation
LPS-stimulated cytokine production of IFN-γ, MCP-1, and IL-10 was attenuated in old BM cells compared to young BM cells. n = 4 per group, *P < 0.05 compared to young BM + LPS.
IFN-γ, interferon-γ; MCP-1, monocyte chemoattractant protein-1; IL, interleukin; TNF-α, tumor necrosis factor-α.
References
1. Korean Statistical Information Service (KOSIS). Future Population Estimates (2015 Population Census) [Internet] Daejeon (Korea): KOSIS; 2016 [cited 2018 Sep 09]. Available from: http://kosis.kr/publication/publicationThema.do?pubcode=PJ
.
2. Cerdá J, Lameire N, Eggers P, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol 2008;3:881–886.


3. Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med 2008;36(4 Suppl):S146–S151.


4. Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 2006;17:1135–1142.


5. Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int 2009;76:1089–1097.


6. Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis 2008;52:262–271.


7. Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 2002;162:2269–2276.


8. O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol 2017;28:407–420.


9. Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol 2015;11:88–101.


10. Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol 2008;109:e102–e107.



11. McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol 2009;21:418–424.



12. Rana A, Murthy B, Pallister Z, et al. Profiling risk for acute rejection in kidney transplantation: recipient age is a robust risk factor. J Nephrol 2017;30:859–868.


13. Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 2009;20:223–228.



14. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–3370.


15. Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 2008;23:842–852.


16. Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 2006;21:1231–1239.


17. Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 2011;22:317–326.



18. Kim MG, Kim SC, Ko YS, Lee HY, Jo SK, Cho W. The role of M2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS One 2015;10:e0143961



19. Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol 2005;77:503–512.


20. Herrero C, Marqués L, Lloberas J, Celada A. IFN-gamma-dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J Clin Invest 2001;107:485–493.



21. Jang HR, Park JH, Kwon GY, et al. Aging has small effects on initial ischemic acute kidney injury development despite changing intrarenal immunologic micromilieu in mice. Am J Physiol Renal Physiol 2016;310:F272–F283.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















