| Kidney Res Clin Pract > Volume 41(3); 2022 > Article |
|
See the article "Risk factors for overcorrection of severe hyponatremia: a post hoc analysis of the SALSA trial" in Volume 41 on page 298.
See "Risk factors for overcorrection of severe hyponatremia: a post hoc analysis of the SALSA trial" at https://doi.org/10.23876/j.krcp.21.180.
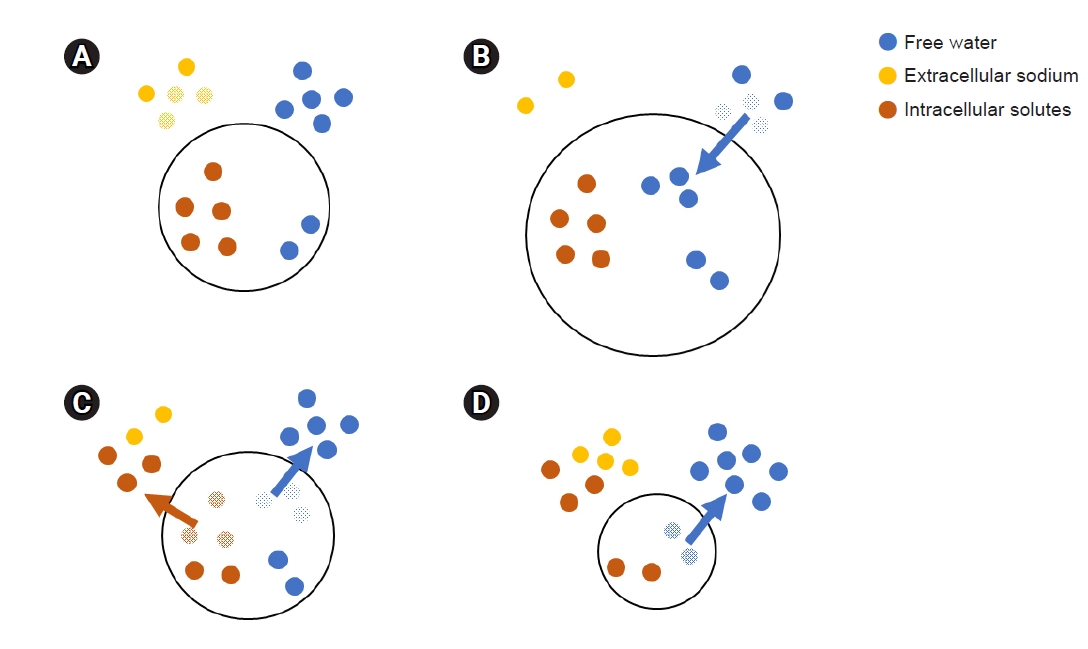
Hyponatremia is common in hospitalized patients [1]. Since serum sodium level is a major determinant of serum osmolality, decreased serum sodium level means that there is a relative excess of free water in the extracellular space (Fig. 1A). This excessive water moves into the cells, causing cellular edema (Fig. 1B) [2]. Hyponatremia is intimately associated with mortality, even in milder forms [3,4]. To date, however, the mechanism behind the increased risk of mortality with hyponatremia is not known [3]. Although acute hyponatremia, hyponatremia which develops within 48 hours, can result in death due to catastrophic brain herniation [5], the hazard of mild chronic hyponatremia cannot be explained by this same mechanism. Therefore, further investigation is needed to determine whether the hazard of mild to moderate chronic hyponatremia is real [3,5].
Over a period of about 48 hours after hyponatremia onset, the brainŌĆÖs cell volume returns to normal by expelling intracellular solutes and water (Fig. 1C) [2]. Despite this adaptation, chronic hyponatremia can result in several symptoms that reflect increased intracranial pressure (e.g., vomiting, stupor, coma, and seizure), which requires urgent management by increasing the serum sodium level [4]. Because intracellular solutes are expelled, overcorrection of the serum sodium level excessively increases serum osmolality to ultimately induce a further out-shift of intracellular water. This can result in cellular shrinkage during management of symptomatic chronic hyponatremia (Fig. 1D). This process is the main pathogenesis of osmotic demyelination syndrome (ODS) [2].
Overcorrection of serum sodium level in symptomatic chronic hyponatremia is the major risk factor of ODS development [2,4]. Since the incidence of ODS is very low, overcorrection has been used as a study outcome (instead of ODS development) in studies for management of symptomatic hyponatremia [1,4,6]. To date, however, there are no universal definitions for hyponatremia overcorrection [4,6,7]. In the SALSA (Efficacy and Safety of Rapid Intermittent Correction Compared With Slow Continuous Correction With Hypertonic Saline In Patients With Moderately Severe or Severe Symptomatic Hyponatremia) trial, a randomized clinical trial for management of symptomatic hyponatremia [1], overcorrection was defined as an increase in serum sodium level by >12 mEq/L or >18 mEq/L within the first 24 or 48 hours, respectively. As a post hoc analysis of the SALSA trial, in this issue, Yang et al. [8] attempted to identify risk factors of overcorrection and to develop a new score called the NASK (hypoNatremia, Alcoholism, Severe symptoms, and hypoKalemia) score to calculate the probability of overcorrection during management of symptomatic hyponatremia. In this study, the variables that were included in the new score were initial serum sodium level and presence of hypokalemia, severe symptoms, and chronic alcoholism (Table 1).
There has only been one prior study to be compared with the study by Yang et al. [8]; Woodfine et al. [6] proposed the SHOR (Severe Hyponatremia Overcorrection Risk) score using data from a single-center retrospective cohort to predict overcorrection during management of hyponatremia. Unlike the study by Yang et al. [8], Woodfine et al. [6] did not have a predefined threshold for overcorrection. Therefore, they had to use a latent class analysis to define overcorrection based on 14 criteria, which resulted in four classes of overcorrection status: none, unlikely, possible, and definite. Using a multinomial instead of a binomial logistic regression, they suggested five risky components (lower initial serum sodium level, hypokalemia, vomiting, somnolence, and low urine osmolality) and three protective components (older age, volume overload, and chest tumor) of overcorrection (Table 1).
As shown in Table 1, some factors were similar, but others were different between the NASK and SHOR scores. Lower initial serum sodium, lower serum potassium, and symptoms suggesting severe signs were robust components to increase the risk of overcorrection. Although chronic alcoholism scored high points on the NASK, it had no role in the SHOR score (Table 1). Although age had no role in the NASK score, older age received negative points in the SHOR score (Table 1). Different clinical and statistical designs, ethnicities, and management strategies likely led to these discordant results. Therefore, future large prospective studies need to be undertaken to validate the usefulness of the NASK and SHOR scores in management of symptomatic chronic hyponatremia.
Although ODS is strongly associated with overcorrection of chronic hyponatremia, other conditions (including alcoholism, malnutrition, and liver transplantation) are also associated with ODS development, even in the absence of hyponatremia or overcorrection [2]. This trend might be due to the control of movement of free water between intracellular and extracellular spaces by serum osmolality and not by serum sodium level. Therefore, any solutes that abruptly increase the extracellular osmolality in the brain can cause ODS. In addition, some conditions (including chronic alcoholism and hypokalemia) can have a synergistic effect on development of ODS, even if the serum sodium level is within the recommended range [4].
What should we watch out for during management of symptomatic chronic hyponatremia? Overcorrection of serum sodium level itself has no clinical role unless it is tightly associated with ODS development. Development of further tools to predict overcorrection during management of hyponatremia will be helpful. However, prediction and prevention of overcorrection are not sufficient. Tailored approaches to treat symptomatic chronic hyponatremia need to be exercised in full consideration of various risk factors of ODS beyond overcorrection.
Figure┬Ā1.
Movement of free water and intracellular solutes according to extracellular sodium level.
(A) Excessive extracellular free water, (B) cellular edema, (C) adaptive expelling, and (D) cellular shrinkage.
Table┬Ā1.
Risk factors for overcorrection in symptomatic hyponatremia
| Factor | NASK score [8] | SHOR score [6] |
|---|---|---|
| Initial sodium level (mEq/L) | Ōēż110: 7 points | <110: 4 points |
| 110ŌĆō115: 4 points | 110ŌĆō112: 2 points | |
| 115ŌĆō120: 2 points | 112ŌĆō114: 1 point | |
| 120ŌĆō125: 0 points | 114ŌĆō116: 0 points | |
| Hypokalemia (K+ <3.0 mEq/L) | 3 points | 1 point |
| Symptom | Severe symptomsa: 3 points | Vomiting: 2 points |
| Somnolence: 2 points | ||
| Chronic alcoholism | 7 points | |
| Age (yr) | 40ŌĆō50: 0 points | |
| 50ŌĆō60: -1 point | ||
| 60ŌĆō70: -2 points | ||
| 70ŌĆō80: -3 points | ||
| 80ŌĆō90: -4 points | ||
| Volume overload | ŌĆō5 points | |
| Urine osmolality, <150 mOsm/kg | 4 points | |
| Chest tumor | ŌĆō5 points | |
| Score range | 0ŌĆō20 points | ŌĆō14 to 13 points |
References
1. Baek SH, Jo YH, Ahn S, et al. Risk of overcorrection in rapid intermittent bolus vs slow continuous infusion therapies of hypertonic saline for patients with symptomatic hyponatremia: the SALSA randomized clinical trial. JAMA Intern Med 2021;181:81ŌĆō92.


3. Hoorn EJ, Zietse R. Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis 2013;62:139ŌĆō149.


4. Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant 2014;29 Suppl 2:i1ŌĆōi39.


5. Kheetan M, Ogu I, Shapiro JI, Khitan ZJ. Acute and chronic hyponatremia. Front Med (Lausanne) 2021;8:693738.



6. Woodfine JD, Sood MM, MacMillan TE, Cavalcanti RB, van Walraven C. Derivation and validation of a novel risk score to predict overcorrection of severe hyponatremia: The Severe Hyponatremia Overcorrection Risk (SHOR) score. Clin J Am Soc Nephrol 2019;14:975ŌĆō982.



-
METRICS

-
- 0 Crossref
- 0 Scopus
- 2,574 View
- 95 Download
- ORCID iDs
-
Hyun Lee Ko

https://orcid.org/0000-0001-9654-8879Sung Woo Lee

https://orcid.org/0000-0002-4419-3938 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















