Coronavirus disease 2019 (COVID-19), the rapidly ongoing pandemic caused by spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), led to a significant impact on transplant systems [
1]. Because of intensified induction therapy as well as maintenance of immune suppressant, there is significant concern about transplantation after COVID-19 infection. Meanwhile, the immune response to SARS-CoV-2 is crucial in detecting primary infection and in confirming postinfection recovery or reinfection [
2]. In this case, we serially monitored humoral and cellular immunity to SARS-CoV-2 in patients who were diagnosed with COVID-19 during preparation for an ABO-incompatible kidney transplantation (ABO icKT) and who successfully received kidney transplantation (KT) without reinfection or complication before or after.
A 47-year-old male patient with chronic kidney disease (stage 5) secondary to diabetic nephropathy for which he was on hemodialysis was planned for a living donor ABO icKT. The potential donor was his spouse, and the isoagglutinin titer was 1:16. According to our center’s protocol, we infused rituximab 4 weeks before KT and planned three plasmapheresis procedures. However, 3 weeks prior to KT, the patient was diagnosed as COVID-19 positive by reverse transcriptase-polymerase chain reaction (SARS-CoV-2 test). Fortunately, the disease course was not severe, and he only required quarantine and symptomatic treatment.
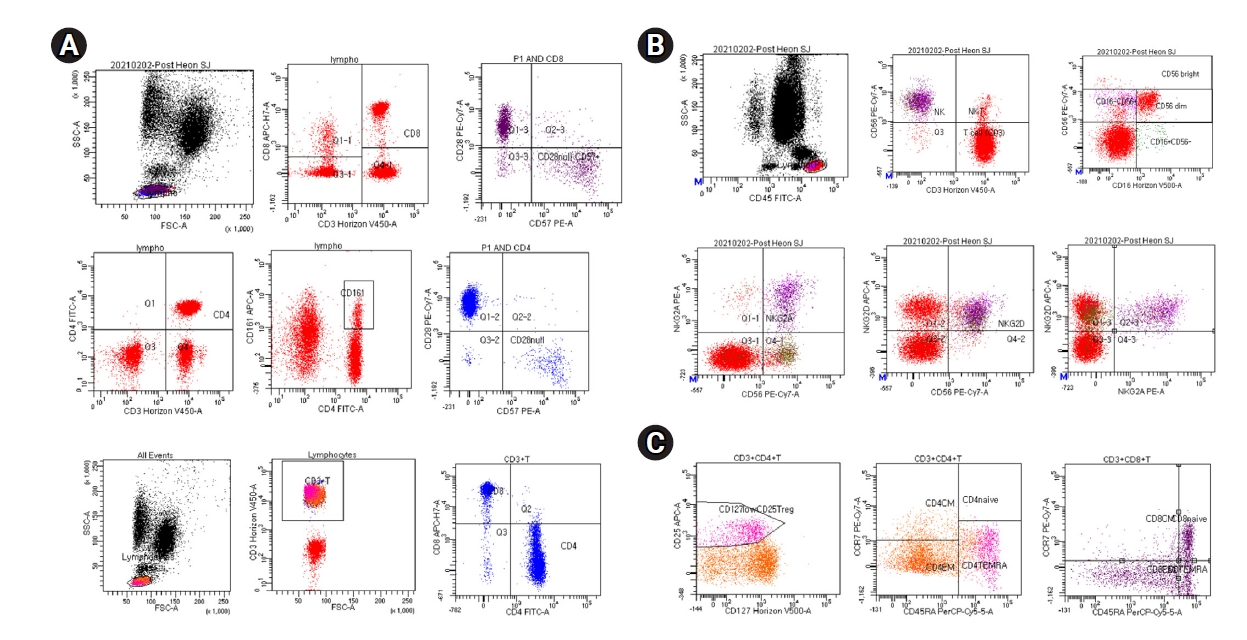
Both humoral and cellular immunity against SARS-CoV-2 had been assessed approximately 2 months prior to KT. Humoral immunity of COVID-19 was detected by measuring anti-SARS-CoV-2 antibodies (Elecsys Anti-SARS-CoV-2 chemiluminescent immunoassay, cutoff of 0.8; Roche Diagnostics, Rotkreuz, Switzerland). That antibody level in serum samples was 0.77 U/mL. The low pre-KT humoral immunity level could be due to the immunosuppressive effect of rituximab for ABO incompatibility. Cellular immunity against SARS-CoV-2 was detected by enzyme-linked immune-spot (ELISPOT, the T-SPOT Discovery SARS-Cov-2 assay kit; Oxford Immunotec, Abingdon-on-Thames, London, UK) and flow cytometry of T cell, regulatory T cell (Treg), and natural killer (NK) subset cytokine expression. Our case was characterized by predominant CD4+ and CD8+ T cell responses, which suggests that he had developed SARS-CoV-2–specific T cell responses [
3] (
Fig. 1). Expression of Treg and NK subset cytokine also was detected. In
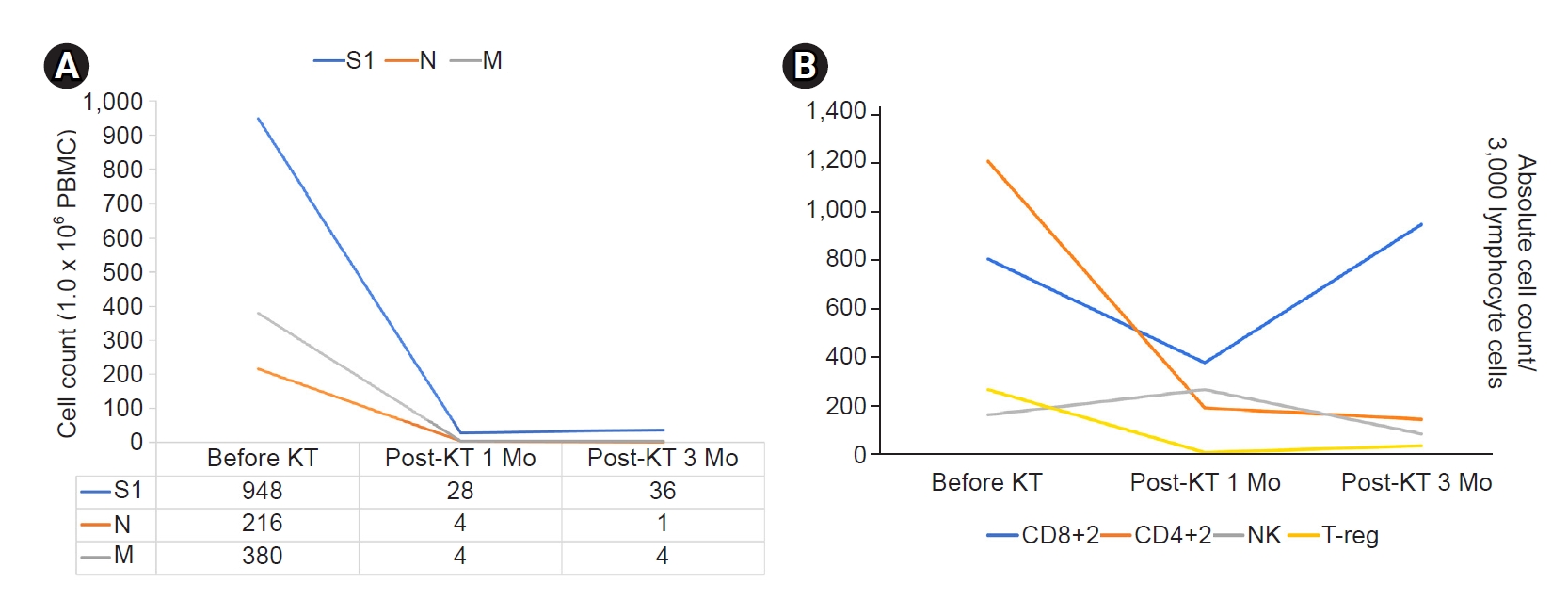
Fig. 2A, adaptive immunity to SARS-CoV-2 virus was confirmed by measurement of SARS-CoV-2–specific T cell immunity (spike protein, N protein, and M protein) by the ELISPOT method.
Therefore, we decided to proceed with the ABO icKT. The patient had received rituximab before the COVID-19 diagnosis, and CD19 and CD20 cell counts in peripheral blood were <0.1%. Hence, the patient underwent only plasmapheresis three times just before KT. Antibody measurement and ELISPOT assay were performed at post-KT intervals of 1 and 3 months. One month after KT, the antibody level was less than 0.4 U/mL, and ELISPOT assay and flow cytometry results also showed decreased cellular immune response (
Fig. 2B). Three months after KT, CD8+ absolute cell count abruptly rebounded, but NK and Treg subset expression remained low. The patient remained stable with an estimated glomerular filtration rate of 104 mL/min/1.73 m
2 up to 10 months posttransplant.
There are approximately four previous case reports of successful transplantation in patients with a mild disease course of COVID-19 and two case reports of such patients with a severe disease course [
4-
8]. No previous case report or cohort study showed adaptive immunity in both precise humoral and cellular patterns. T cell-mediated immune response is required for protection against SARS-CoV-2 and is a surrogate of acquired immune protection from reinfection [
2]. Though detected humoral immunity value was below the cutoff value, we verified adaptive immunity by detection of more precise cellular immunity with ELISPOT.
In conclusion, our data are reassuring that functional SARS-CoV-2–specific T cell responses are acquired as soon as 6 weeks after initial COVID-19 diagnosis and retained for 6 months following infection. This suggests that adaptive T cell immunity is sustained, and the ideal wait time for transplant could be as soon as only 6 weeks after initial diagnosis of COVID-19. Most KT recipients slowly recover their immune status as immunosuppressant dosages are tapered over time. Our patient recovered his immune status, and CD8+ expression level was normal at 3 months after KT. This case is promising for patients who have suffered from COVID-19 before KT and suggests that recovery from COVID-19 and the presence of T cell immunity against SARS-CoV-2 could be a surrogate marker for safe and successful KT.










 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















