| Kidney Res Clin Pract > Volume 39(3); 2020 > Article |
|
Abstract
Background
Methods
Results
Conclusion
Acknowledgments
Notes
Conflicts of interest
The authors declare that no conflict of interest exists. The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the National Institute for Health Research (NIHR), or the Department of Health.
Funding
This research was part-funded by the Stoneygate Trust and supported by the National Institute for Health Research Leicester Biomedical Research Centre. At the time of writing this manuscript, Emma L Watson was supported by a Kidney Research UK Post-Doctoral Fellowship.
AuthorsŌĆÖ contributions
Emma L. Watson, Jo├Żo L. Viana, and Alice C. Smith contributed to the original conception and design of the study. Thomas J. Wilkinson contributed to conception of the current retrospective analysis. All authors contributed to the acquisition, analysis, or interpretation of data. Thomas J. Wilkinson drafted the manuscript. All authors critically revised the manuscript, gave final approval, and agree to be accountable for all aspects of the work to ensure integrity and accuracy.
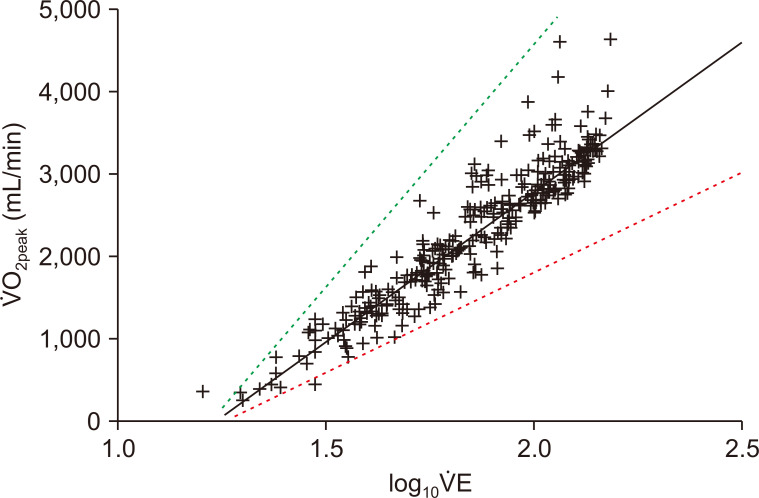
Figure┬Ā1
Example relationship between oxygen uptake (Ō®ÆO2) and log10 transformed total ventilation (Ō®ÆE) during incremental cardiopulmonary exercise testing in a 59-year-old male with chronic kidney disease (solid black line).
Figure┬Ā2
Mean changes in oxygen uptake efficiency slope (OUES)

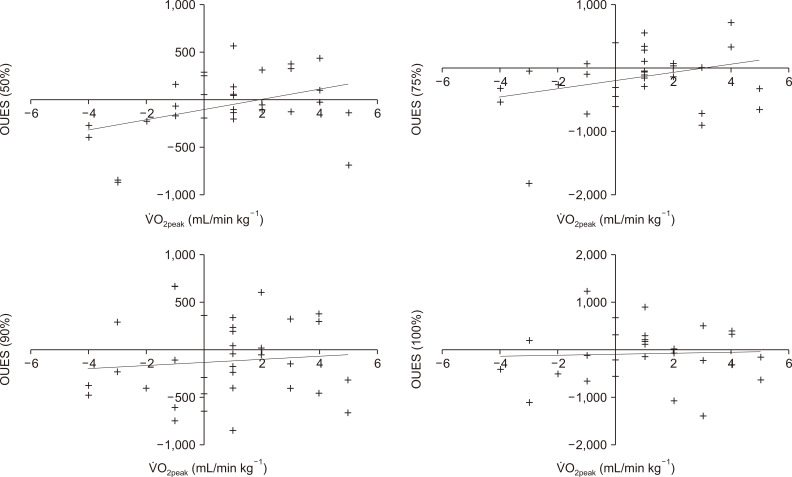
Figure┬Ā3
Association between changes pre- to post-exercise in oxygen uptake and oxygen uptake efficiency slope (OUES) calculated at 100%, 90%, 75%, and 50% of exercise duration across all 32 patients.
Table┬Ā1
| Characteristic | Value |
|---|---|
| Age (yr) | 61.1 ┬▒ 12.1 |
| Sex (female) | 18 (56.3) |
| Ethnicity | |
| White British | 22 (68.8) |
| South Asian | 9 (28.1) |
| Other | 1 (3.1) |
| eGFR (mL/min)a | 25.3 ┬▒ 7.4 |
| Stage 3b | 9 (28.1) |
| Stage 4 | 21 (65.6) |
| Stage 5 | 1 (3.1) |
| Etiology | |
| Unknown | 18 (56.3) |
| IgA nephropathy | 3 (9.4) |
| Diabetic nephropathy | 3 (9.4) |
| PKD | 2 (6.3) |
| Other | 6 (18.8) |
| Body mass index (kg/m2) | 30.1 ┬▒ 6.0 |
| Hemoglobin (g/L) | 119.4 ┬▒ 15.1 |
| Albumin (g/L) | 40.8 ┬▒ 2.9 |
| Hypertension | 15 (46.9) |
| Diabetes | 7 (21.9) |
| Cardiovascular disease | 4 (12.5) |
| Blood pressure (systolic/diastolic) (mmHg) | 130.6 ┬▒ 18.9/72.0 ┬▒ 11.6 |
Table┬Ā2
| 6-week control period | 12-week exercise intervention | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Baseline | Pre-exercise | Δ (baseline to pre-exercise) (95% CI) | P | Post-exercise | Δ (pre- to post-exercise) (95% CI) | P | ||
| Ō®ÆO2peak (L/min) | 1.6 ┬▒ 0.6 | 1.7 ┬▒ 0.5 | 0.0 (-0.1 to 0.1) | 0.377 | 1.7 ┬▒ 0.5 | 0.0 (0.0 to 0.1) | 0.239 | |
| Ō®ÆO2peak (mL/min kg-1) | 19.4 ┬▒ 5.8 | 20.1 ┬▒ 5.5 | 0.8 (-0.3 to 1.8) | 0.167 | 21.0 ┬▒ 5.7 | 0.8 (0.0 to 1.7) | 0.057 | |
| OUES (100%) | 2,173.1 ┬▒ 667.8 | 2,181.1 ┬▒698.0 | 8.1 (-212.1 to 228.2) | 0.941 | 2,096.3 ┬▒ 617.9 | -84.8 (-285.7 to 116.0) | 0.396 | |
| OUES (90%) | 2,156.9 ┬▒ 633.9 | 2,204.8 ┬▒ 642.3 | 48.0 (-138.6 to 234.6) | 0.603 | 2,084.9 ┬▒ 566.3 | -119.9 (-264.5 to 24.7) | 0.101 | |
| OUES (75%) | 2,051.5 ┬▒ 633.9 | 2,152.9 ┬▒ 639.8 | 101.4 (-96.0 to 298.7) | 0.303 | 1,981.0 ┬▒ 484.5 | -171.9 (-349.4 to 5.6) | 0.057 | |
| OUES (50%) | 1,948.8 ┬▒ 579.4 | 2,008.3 ┬▒ 492.6 | 59.5 (-108.5 to 227.6) | 0.476 | 1,962.4 ┬▒ 438.9 | -45.9 (-165.7 to 74.0) | 0.441 | |
| Peak Ō®ÆE/Ō®ÆO2 (AU) | 40.7 ┬▒ 8.4 | 42.3 ┬▒ 8.4 | 1.7 (-0.1 to 3.4) | 0.059 | 43.5 ┬▒ 8.0 | 1.2 (-0.4 to 2.8) | 0.148 | |
| Peak Ō®ÆE/Ō®ÆCO2 (AU) | 34.8 ┬▒ 6.7 | 34.9 ┬▒ 5.0 | 0.2 (-1.0 to 1.3) | 0.797 | 35.1 ┬▒ 5.6 | 0.2 (-0.7 to 1.1) | 0.626 | |
| Peak Ō®ÆE (L) | 70.5 ┬▒ 27.2 | 74.3 ┬▒ 24.7 | 3.8 (-1.5 to 9.1) | 0.153 | 79.2 ┬▒ 20.2 | 4.8 (-0.5 to 9.2) | 0.031* | |
| Peak RER (Ō®ÆO2/Ō®ÆCO2) | 1.20 ┬▒ 0.10 | 1.20 ┬▒ 0.15 | 0.03 (-0.02 to 0.08) | 0.288 | 1.24 ┬▒ 0.10 | 0.40 (0.00 to 0.10) | 0.042* | |
| Peak HR (beats/min)a | 136.7 ┬▒ 21.5 | 139.1 ┬▒ 21.4 | 2.5 (-3.0 to 7.9) | 0.360 | 137.7 ┬▒ 19.5 | -1.4 (-6.3 to 3.5) | 0.568 | |
| Peak RR (breath/min) | 37.4 ┬▒ 9.5 | 39.3 ┬▒ 8.5 | 1.8 (-0.3 to 3.9) | 0.086 | 42.4 ┬▒ 8.7 | 3.1 (1.1 to 5.2) | 0.003* | |
| Peak VT (AU) | 1.9 ┬▒ 0.6 | 1.9 ┬▒ 0.6 | 0.0 (-0.1 to 0.1) | 0.398 | 1.9 ┬▒ 0.6 | 0.0 (-0.1 to 0.1) | 0.644 | |
| Duration (sec) | 373.4 ┬▒ 185.1 | 372.4 ┬▒ 161.9 | -1.0 (-42.4 to 40.5) | 0.962 | 413.3 ┬▒ 155.5 | 40.9 (16.0 to 65.8) | 0.002* | |
| Body mass (kg) | 83.5 ┬▒ 17.2 | 82.8 ┬▒ 16.4 | -0.7 (-1.5 to 0.1) | 0.087 | 82.4 ┬▒ 17.3 | -0.4 (-1.3 to 0.4) | 0.312 | |
Table┬Ā3
| Pre-exercise to post-exercise intervention (12 weeks) | ||||
|---|---|---|---|---|
|
|
||||
| AE group (n=15) | CE group (n=17) | Pa | ||
| Ō®ÆO2peak (L/min) | Pre-exercise | 1.8 ┬▒ 0.6 | 1.5 ┬▒ 0.4 | |
| Post-exercise | 1.9 ┬▒ 0.5 | 1.5 ┬▒ 0.3 | ||
| Δ (95% CI) | 0.1 (-0.1 to 0.2) | 0.0 (-0.1 to 0.1) | 0.098 | |
| P | 0.275 | 0.581 | ||
| Ō®ÆO2peak (mL/min kg-1) | Pre-exercise | 21.2 ┬▒ 6.5 | 19.2 ┬▒ 4.5 | |
| Post-exercise | 22.1 ┬▒ 6.5 | 19.9 ┬▒ 4.7 | ||
| Δ (95% CI) | 0.9 (-0.4 to 2.2) | 0.8 (-0.5 to 2.1) | 0.602 | |
| P | 0.145 | 0.232 | ||
| OUES (100%) | Pre-exercise | 2,301.4 ┬▒ 609.8 | 2,075.0 ┬▒ 770.1 | |
| Post-exercise | 2,163.8 ┬▒ 665.9 | 2,036.7 ┬▒ 586.3 | ||
| Δ (95% CI) | -137.6 (-478.8 to 203.6) | 38.3 (-302.6 to 226.0) | 0.642 | |
| P | 0.402 | 0.763 | ||
| OUES (90%) | Pre-exercise | 2,458.4 ┬▒ 562.1 | 1,981.0 ┬▒ 639.7 | |
| Post-exercise | 2,199.5 ┬▒ 599.1 | 1,983.8 ┬▒ 533.0 | ||
| Δ (95% CI) | -259.0 (-487.4 to 30.5) | 2.8 (-181.5 to 187.1) | 0.250 | |
| P | 0.029* | 0.975 | ||
| OUES (75%) | Pre-exercise | 2,318.0 ┬▒ 529.7 | 2,007.2 ┬▒ 706.7 | |
| Post-exercise | 2,136.1 ┬▒ 535.6 | 1,844.1 ┬▒ 401.5 | ||
| Δ (95% CI) | -181.9 (-409.5 to 45.8) | -163.1 (-454.6 to 128.4) | 0.196 | |
| P | 0.109 | 0.253 | ||
| OUES (50%) | Pre-exercise | 2,235.4 ┬▒ 433.3 | 1,807.9 ┬▒ 463.6 | |
| Post-exercise | 2,110.8 ┬▒ 415.9 | 1,831.6 ┬▒ 427.8 | ||
| Δ (95% CI) | -124.6 (-293.5 to 44.2) | -163.1 (-454.6 to 128.4) | 0.869 | |
| P | 0.136 | 0.253 | ||
| Peak Ō®ÆE/Ō®ÆO2 (AU) | Pre-exercise | 39.9 ┬▒ 5.7 | 44.5 ┬▒ 9.9 | |
| Post-exercise | 41.4 ┬▒ 5.1 | 45.3 ┬▒ 9.7 | ||
| Δ (95% CI) | 1.6 (-0.9 to 4.0) | 0.8 (-1.6 to 3.2) | 0.853 | |
| P | 0.191 | 0.471 | ||
| Peak Ō®ÆE/Ō®ÆCO2 (AU) | Pre-exercise | 33.7 ┬▒ 4.0 | 36.0 ┬▒ 5.7 | |
| Post-exercise | 34.3 ┬▒ 5.0 | 35.9 ┬▒ 6.2 | ||
| Δ (95% CI) | 0.6 (-0.7 to 1.8) | -0.1 (-1.5 to 1.3) | 0.615 | |
| P | 0.327 | 0.858 | ||
| Peak Ō®ÆE (L) | Pre-exercise | 75.1 ┬▒ 23.0 | 26.8 ┬▒ 6.5 | |
| Post-exercise | 83.2 ┬▒ 18.7 | 75.6 ┬▒ 21.4 | ||
| Δ (95% CI) | 8.0 (1.1 to 15.0) | 2.0 (-3.8 to 7.8) | 0.095 | |
| P | 0.026* | 0.478 | ||
| Peak RER (Ō®ÆO2/Ō®ÆCO2) | Pre-exercise | 1.16 ┬▒ 0.17 | 1.23 ┬▒ 0.12 | |
| Post-exercise | 1.23 ┬▒ 0.08 | 1.26 ┬▒ 0.11 | ||
| Δ (95% CI) | 0.07 (-0.03 to 0.17) | 0.03 (-0.01 to 0.01) | 0.864 | |
| P | 0.144 | 0.122 | ||
| Peak HR (beats/min)b | Pre-exercise | 145.3 ┬▒ 21.0 | 133.8 ┬▒ 21.0 | |
| Post-exercise | 144.3 ┬▒ 18.4 | 132.1 ┬▒ 19.2 | ||
| Δ (95% CI) | -1.0 (-9.2 to 7.2) | -1.7 (-8.6 to 5.2) | 0.442 | |
| P | 0.794 | 0.601 | ||
| Peak RR (breath/min) | Pre-exercise | 39.5 ┬▒ 9.1 | 39.0 ┬▒ 8.2 | |
| Post-exercise | 43.0 ┬▒ 7.8 | 41.9 ┬▒ 9.7 | ||
| Δ (95% CI) | 3.5 (-0.4 to 7.3) | 2.9 (0.6 to 5.2) | 0.627 | |
| P | 0.073 | 0.017* | ||
| Peak VT (AU) | Pre-exercise | 2.0 ┬▒ 0.7 | 1.9 ┬▒ 0.5 | |
| Post-exercise | 2.0 ┬▒ 0.7 | 1.9 ┬▒ 0.5 | ||
| Δ (95% CI) | 0.0 (-0.18 to 0.15) | 0.0 (-0.1 to 0.1) | 0.736 | |
| P | 0.873 | 0.593 | ||
| Duration (sec) | Pre-exercise | 412.5 ┬▒ 193.1 | 337.1 ┬▒ 123.7 | |
| Post-exercise | 457.4 ┬▒ 165.9 | 374.5 ┬▒ 139.0 | ||
| Δ (95% CI) | 44.9 (6.2 to 83.5) | 37.4 (1.1 to 73.7) | 0.153 | |
| P | 0.026* | 0.044* | ||
╬ö, difference; AE group, aerobic exercise group; AU, arbitrary units; CI, confidence interval; CE group, combined exercise group; HR, heart rate; OUES, oxygen uptake efficiency slope; RER, respiratory exchange ratio; RR, respiratory rate; V╠ćE, minute ventilation; V╠ćO2, oxygen consumption; VT, ventilatory threshold.
Table┬Ā4
| Baseline | Pre-exercise | Post-exercise | |
|---|---|---|---|
| Mean ┬▒ SD | |||
| OUES (100%) | 2,173.1 ┬▒ 667.8 | 2,181.1 ┬▒ 698.0 | 2,096.3 ┬▒ 617.9 |
| OUES (90%) | 2,156.9 ┬▒ 633.9 | 2,204.8 ┬▒ 642.3 | 2,084.9 ┬▒ 566.3 |
| OUES (75%) | 2,051.5 ┬▒ 633.9 | 2,152.9 ┬▒ 639.8 | 1,981.0 ┬▒ 484.5 |
| OUES (50%) | 1,948.8 ┬▒ 579.4 | 2,008.3 ┬▒ 492.6 | 1,962.4 ┬▒ 438.9 |
| Inter-item coefficient r | |||
| OUES (50%) vs. (75%) | 0.896 | 0.810 | 0.851 |
| OUES (50%) vs. (90%) | 0.797 | 0.714 | 0.790 |
| OUES (50%) vs. (100%) | 0.747 | 0.668 | 0.787 |
| OUES (75%) vs. (90%) | 0.857 | 0.788 | 0.879 |
| OUES (75%) vs. (100%) | 0.812 | 0.865 | 0.790 |
| OUES (90%) vs. (100%) | 0.830 | 0.873 | 0.764 |
| CronbachŌĆÖs ╬▒ (r) | 0.948 | 0.934 | 0.937 |
| P | < 0.001* | < 0.001* | < 0.001* |
References
- TOOLS
-
METRICS

- Related articles
-
Pharmacologic therapeutics in sarcopenia with chronic kidney disease2024 March;43(2)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















