| Kidney Res Clin Pract > Volume 37(3); 2018 > Article |
|
Abstract
Brucellosis is the most common zoonotic disease in Greece, with an endemic distribution and can affect any organ. Infiltration of the renal parenchyma causes acute and chronic interstitial nephritis with granulomas, whereas renal glomeruli are rarely affected. The disease has been sporadically reported, and it causes various histopathologic patterns. Herein, we describe the case of a 39-year-old stock breeder with a history of recurrent episodes of bacteremia caused by Brucella melitensis over a period of 3 years. Two months after the last episode of bacteremia, he presented with mild renal insufficiency, nephrotic range proteinuria, and microscopic hematuria. A renal biopsy revealed membranoproliferative glomerulonephritis with a pattern of focal-segmental nodular sclerosis and moderate tubulointerstitial fibrosis. The patient received antimicrobial and corticosteroid therapy with partial remission of the nephrotic syndrome.
Brucellosis is a common, endemic zoonosis with a worldwide distribution. The main pathogenic strain in humans is Brucella melitensis, although infection with other strains has been sporadically reported. B. melitensis is usually transmitted by digestion of contaminated food and rarely by direct contact through the skin or by inhalation. The bacterium enters the lymphatic system, passes to the bloodstream, and invades various organs, most commonly the bones, liver, and spleen as well as any other organ [1]. The course of the disease can be acute, subacute, or chronic (lasting more than 12 months). Common clinical manifestations include malaise, fever, back pain, sweat, and weight loss. Arthritis, hepatosplenomegaly, orchitis, endocarditis, abscesses, and pleural effusions may occur during the course of the disease depending on the affected organ. Isolation of the bacteria in blood or tissue cultures can confirm the diagnosis, whereas serological tests are useful for both diagnosis and monitoring of the disease. The duration and type of treatment depend on location and severity of the disease and usually involves a combination of antibiotics [2].
Although brucellae can be found in the urine of patients with acute disease [3], direct renal infection is uncommon. Glomerulonephritis associated with brucellosis has rarely been reported in the literature. Herein, we present the case of a 39-year-old patient with chronic brucellosis who developed membranoproliferative glomerulonephritis.
A 39-year-old farmer was referred to our nephrology department for nephrotic syndrome and gross proteinuria (8,782 mg/day). Two months prior to the onset of his symptoms, he was diagnosed with bacteremia caused by B. melitensis and received antibiotic treatment with doxycycline, rifampin, and trimethoprim/sulfamethoxazole. His medical history included recurrent episodes of brucella bacteremia without organ involvement over the previous three years. He had no other infectious or chronic diseases. Interestingly, there was no evidence of renal involvement during the first episode of brucella infection, as the urinalysis was unremarkable without hematuria, pyuria, or proteinuria.
On admission, the patient was afebrile, and his blood pressure was 145/92 mmHg. Clinical examination revealed periorbital and pedal edema, hepatomegaly, and no other abnormal findings. Laboratory tests are summarized in Table 1, including the Brucella serum agglutination test (SAT), which was performed two months prior to admission, on admission, and during follow-up. The Brucella SAT was performed in separate tubes by incubating a standardized volume and concentration of whole Brucella cell suspension with a standardized volume of the patientŌĆÖs serum in doubling dilutions ranging from 1:20 to 1:1,280. The tubes were incubated at 37┬░C for 24 hours in a water bath and then were examined visually. The highest serum dilution showing more than 50% agglutination was considered the agglutination titer. Serum protein electrophoresis did not detect a monoclonal fraction. Virological tests for hepatitis viruses, cytomegalovirus, Epstein-barr virus, herpes simplex virus 1 and 2, and Toxoplasma gondii were negative, and immunological tests revealed low complement levels and a positive (qualitative) cryoglobulin titer. Ultrasonographic imaging of the kidneys revealed no urinary abnormalities, and vegetations were absent in the echocardiogram.
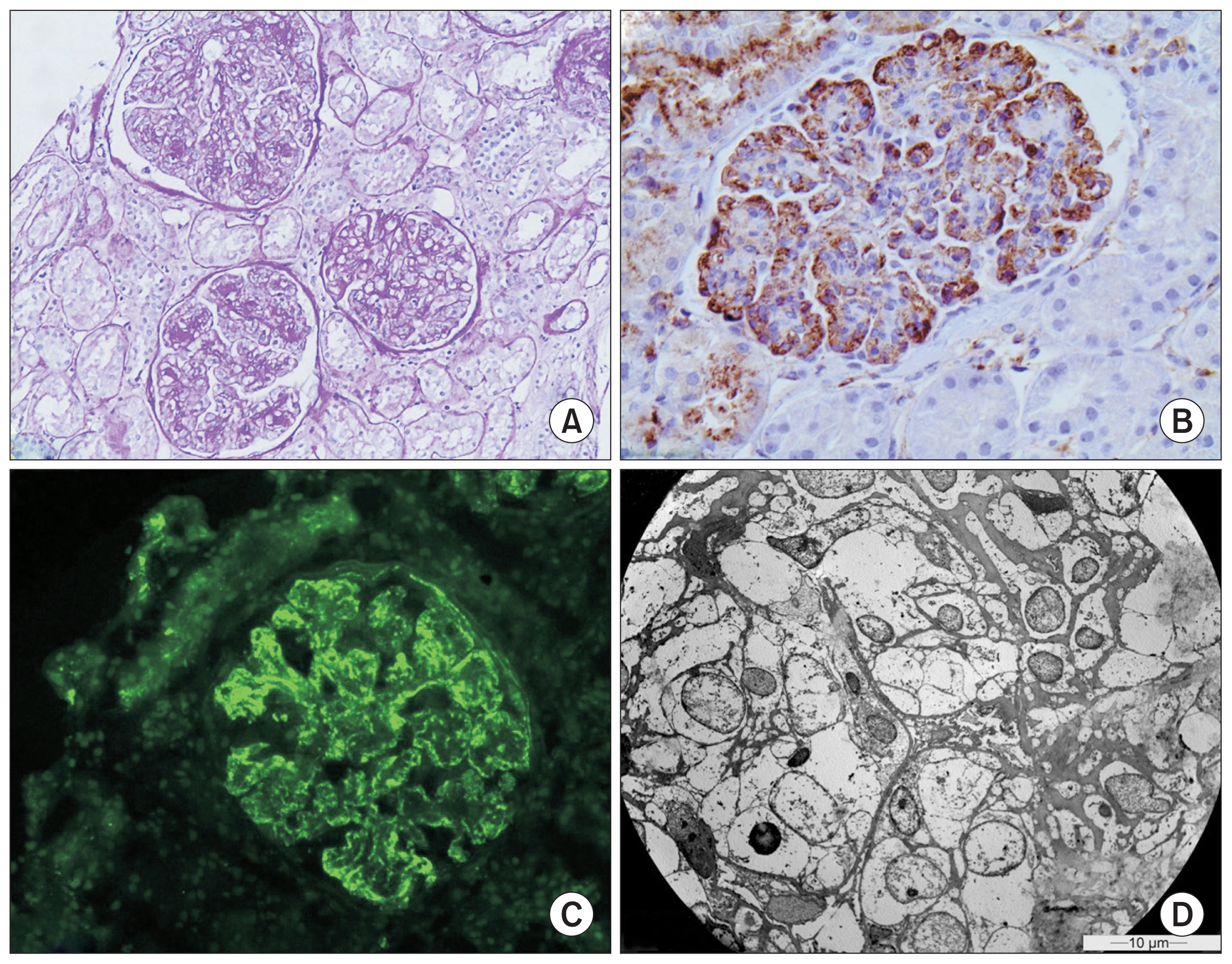
The patient received the appropriate conservative treatment for nephrotic syndrome, consisting of an angiotensin receptor blocker at the maximum tolerated dose and a statin. Subsequently, a percutaneous renal biopsy was performed and revealed membranoproliferative glomerulonephritis (Fig. 1A). Pathology showed diffuse global intercapillary and mesangial hyperplasia, glomerular basement membrane thickening and duplication, podocyte hypertrophy and edema, narrowing of capillary lumens, and inflammatory infiltrations. There was moderate tubulointerstitial atrophy and fibrosis (15ŌĆō20%) as well as vascular intima thickening and edema. Immunofluorescence showed intense C3, C4, and immunoglobulin M staining with granular peripheral subendothelial and mesangial deposits, whereas staining of other immunoglobulins, as well as ╬║- and ╬╗-chains, was moderate (Fig. 1B, C). New basement membrane formation and mesangial matrix expansion were evident by electron microscopy, along with obliteration of capillary lumens by inflammatory cells, extensive elimination of podocyte foot processes, and dense mesangial and subendothelial deposits (Fig. 1D). The biopsy did not reveal any granulomas or findings consistent with cryoglobulinemic glomerulonephritis.
The patient remained on antibiotic and antihypertensive therapy, but five months later presented with worsened edema, deregulated arterial blood pressure, and persistent proteinuria of 14 g/day. Subsequently, oral prednisolone at 0.5 mg/kg daily was administered. Fifteen days after initiation of treatment, the patient showed significant clinical improvement with resolution of edema. Additionally, a partial remission of proteinuria (4 g/day) was documented. Approximately two months after corticosteroid initiation, his clinical course was complicated by bacterial pneumonia that was treated successfully with intravenous antibiotics for 14 days. Having completed a three-month course of prednisolone, the patient underwent a fast dose tapering of corticosteroids until total withdrawal of the drug. In subsequent follow-up visits, the patient had stable renal function (serum creatinine, 1.2 mg/dL), but his proteinuria relapsed (600 mg/dL, urine spot). Twenty-eight months after renal biopsy, he shows mildly worsened kidney function (estimated glomerular filtration rate, 47 mL/min/1.73 m2) with persistent high-grade proteinuria while maintaining conservative treatment.
Renal involvement in brucellosis has been known since 1889 when David Bruce [4] reported the presence of proteinuria in severely affected patients. Later, Eyre et al [5] observed that septic patients with acute brucellosis developed acute nephritis with oliguria and albuminuria, further identifying the prevalence of albuminuria at 8%. In the following years, sporadic case reports of patients with brucellosis, endocarditis, and acute nephritis were documented in which the kidneys were grossly enlarged with microabscesses and granulomas [6]. The main pathogenic mechanism of kidney damage was originally considered to be direct invasion of bacteria in the renal parenchyma.
The first direct indications that kidney damage in brucellosis might be immune-mediated emerged as a result of the extensive study by Margolis et al [7], who demonstrated the presence of glomerulonephritis in 4 animals with brucellosis. Since then, a small number of publications have described the histological findings of tubulointerstitial and glomerular lesions in patients with brucellosis [8]. This evidence has recently led to the histological classification of renal brucellosis in three types; acute interstitial nephritis and pyelonephritis, chronic interstitial nephritis with granuloma formation, and glomerulonephritis associated with vasculitis and/or endocarditis [9]. Moreover, a fourth type has been proposed for cases involving formation of renal abscesses [3].
Membranoproliferative glomerulonephritis in conjunction with brucellosis is only described in 4 cases in the literature (Table 2) [8,10ŌĆō12]. These patients were all males with a mean age of 41 years (range, 28ŌĆō61 years). Brucella infection was confirmed by serological methods or blood and tissue cultures. At diagnosis, all patients had microscopic hematuria, and only 2 had nephrotic-range proteinuria. Management consisted of antibiotic therapy including rifampin and doxycycline in 3 patients. Additionally, corticosteroid treatment was initiated in 2 patients, whereas cyclosporine was added in one patient who had nephrotic range proteinuria. All patients showed a favorable outcome with partial or complete recovery and retained satisfactory kidney function during follow-up.
Our patient had differences and similarities to the case reports described in the literature. First, he presented with typical nephrotic syndrome and mild renal impairment due to membranoproliferative glomerulonephritis. His clinical and laboratory findings were in agreement with the available literature, although cases of nephritic syndrome [10] and rapidly progressive glomerulonephritis with red blood cell casts [11] have been reported. Similar to 3 of the 4 cases, our patient had no organ involvement, whereas central nervous system involvement was described in one patient [8]. On the contrary, the presence of cryoglobulinemia was not recorded in any of the aforementioned cases. It is noteworthy that 4 cases of brucellosis and systemic vasculitis with cryoglobulinemia have been reported [13,14]. Of them, only one patient had impaired renal function with microscopic hematuria and proteinuria, but a renal biopsy was not performed. In our patient, the presence of cryoglobulinemia was considered a sequela of chronic infection.
It is worth mentioning that, while the course of disease was acute or subacute (duration < 1 year) in the majority of cited cases, our patient had a confirmed, chronic, active brucella infection at least three years prior to the onset of glomerulopathy. In all these patients, including ours, diagnosis was based on positive SAT and/or blood cultures. Only one of the presented patients had a history of acute brucellosis 28 years prior to the appearance of membranoproliferative glomerulonephritis; however, SAT and blood and tissue cultures at the time of renal involvement were all negative; therefore, an association between the two conditions was considered after exclusion of all other possible causes [12]. Membranoproliferative glomerulonephritis often develops secondary to chronic infections, most frequently hepatitis C infection, and is considered of immunologic origin [15]. Even though brucellosis is not generally considered a common infectious cause of membranoproliferative glomerulonephritis, it should be considered in cases of chronic infections with frequent relapses or limited response to antibiotic treatment.
Regarding treatment, our patient was administered corticosteroids 5 months after diagnosis due to resistant nephrotic syndrome and lack of responsiveness to antibiotic treatment. In two of the cited cases, corticosteroids were started at diagnosis [11,12], and cyclosporine was subsequently added in one patient after a relapse of proteinuria [12]. The remaining two cases received only antibiotics [8,10]. The outcome of our patient is considered the worst of all the cases described in the literature, as he continues to show persistent proteinuria and deteriorating renal function after 28 months of follow-up. Unlike the other patients, corticosteroid treatment in our patient was beneficial in the short term but at the cost of serious infectious side effects. Of the previously described patients, three had a short follow-up with renal function recovery [8,11] and low-grade proteinuria [10]. Only one patient had a follow-up longer than three years, during which he remained in partial remission of nephrotic syndrome [12].
In conclusion, although rare as a disease entity, glomerulonephritis associated with brucellosis should always be considered during diagnostic evaluation of a patient with proteinuria, microscopic hematuria, and a relevant medical history. Moreover, it would be prudent to monitor markers of kidney damage in patients with a history of brucellosis, since renal involvement may appear years later. Immunosuppressive treatment and antibiotics could be recommended in selected cases in order to halt the ongoing immune disorder, resulting in a favorable outcome.
Figure┬Ā1
Renal biopsy findings
(A) Glomerulonephritis with membranoproliferative pattern with endocapillary and mesangial proliferation (periodic acidŌĆōSchiff, ├Ś200). (B) Positive immunohistochemical staining for immunoglobulin M with peripheral distribution (├Ś400). (C) Strong (++++) granular deposition of C3 complement with subendothelial and mesangial localization (├Ś200). (D) Obstruction of the capillary lumen by endothelial, mesangial, and inflammatory cells (├Ś1,400).
Table┬Ā1
Laboratory tests at diagnosis, beginning of steroid treatment, and at follow-up
Table┬Ā2
Main characteristics, treatment options, and outcomes of patients with brucellosis and membranoproliferative glomerulonephritis
| Country | Duration of Brucella infection | Age (yr) | Sex | eGFR (mL/min) | Proteinuria (g/24 hr) | Hematuria/pyuria | Endocarditis | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Turkey | NA | 41 | Male | 9.4 | 3.0 | Yes/No | No | RIF + DOX | Recovery | Ceylan et al [8] |
| Turkey | NA | 33 | Male | 69.0 | 1.4 | Yes/No | No | RIF + DOX | Partial recovery | Altiparmak et al [10] |
| Iran | 4 mo | 28 | Male | 19.1 | 1.0 | Yes/No | No | RIF + DOX + Cs | Recovery | Ardalan and Shoja [11] |
| Poland | 28 yr prior | 61 | Male | NA | 8.29 | Yes/No | No | Cs + CsA | Partial recovery | Kusztal et al [12] |
References
1. Colmenero JD, Reguera JM, Martos F, et al. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore) 75:195ŌĆō211. 1996;


2. Young EJ. Brucellosis: current epidemiology, diagnosis, and management. Curr Clin Top Infect Dis 15:115ŌĆō128. 1995;

3. Bakri FG, Wahbeh A, Mahafzah A, Tarawneh M. Brucella glomerulonephritis resulting in end-stage renal disease: a case report and a brief review of the literature. Int Urol Nephrol 40:529ŌĆō533. 2008;


5. Eyre JWH, Durh MS, Cantab DPH. The milroy lectures on melitensis septic├”mia (Malta or Mediterranean fever). Lancet 171:1747ŌĆō1752. 1908;

6. Madkour MM. Genitourinary brucellosis. In: Madkour MM, Brucellosis. London: Butterworth-Heinemann; p. 152ŌĆō159. 1989.

7. Margolis G, Forbus WD, Kerby GP, Lide TN. Glomerulonephritis occurring in experimental brucellosis in dogs. Am J Pathol 23:983ŌĆō993. 1947;


8. Ceylan K, Karahocagil MK, Soyoral Y, et al. Renal involvement in Brucella infection. Urology 73:1179ŌĆō1183. 2009;


9. Elzouki AY, Akthar M, Mirza K. Brucella endocarditis associated with glomerulonephritis and renal vasculitis. Pediatr Nephrol 10:748ŌĆō751. 1996;


10. Altiparmak MR, Pamuk GE, Pamuk ON, Tabak F. Brucella glomerulonephritis: review of the literature and report on the first patient with brucellosis and mesangiocapillary glomerulonephritis. Scand J Infect Dis 34:477ŌĆō480. 2002;


11. Ardalan MR, Shoja MM. Rapidly progressive glomerulonephritis in a patient with brucellosis. Nephrol Dial Transplant 21:1743ŌĆō1744. 2006;


12. Kusztal M, Dorobisz A, Kuzniar J, et al. Dissecting aneurysm of the thoracic aorta in a patient with nephrotic syndrome and brucellosis. Int Urol Nephrol 39:641ŌĆō645. 2007;


13. Hermida Lazcano I, S├Īez M├®ndez L, Solera Santos J. Mixed cryoglobulinemia with renal failure, cutaneous vasculitis and peritonitis due to Brucella melitensis. J Infect 51:e257ŌĆōe259. 2005;





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















