| Kidney Res Clin Pract > Volume 36(1); 2017 > Article |
|
Abstract
Background
Many epidemiologic studies have reported on the controversial concept of the obesity paradox. The presence of acute kidney injury (AKI) can accelerate energy-consuming processes, particularly in patients requiring continuous renal replacement therapy (CRRT). Thus, we aimed to investigate whether obesity can provide a survival benefit in this highly catabolic condition.
Methods
We conducted an observational study in 212 patients who had undergone CRRT owing to various causes of AKI between 2010 and 2014. The study end point was defined as death that occurred within 30 days after the initiation of CRRT.
Results
Patients were categorized into three groups according to tertiles of body mass index (BMI). During ≥30 days after the initiation of CRRT, 39 patients (57.4%) in the highest tertile died, as compared with 58 patients (78.4%) in the lowest tertile (P = 0.02). In a multivariable analysis adjusted for cofounding factors, the highest tertile of BMI was significantly associated with a decreased risk of death (hazard ratio [HR], 0.57; 95% confidence interval [CI], 0.37–0.87; P = 0.01). This significant association remained unaltered for 60-day (HR, 0.64; 95% CI, 0.43–0.94; P = 0.03) and 90-day mortality (HR, 0.66; 95% CI, 0.44–0.97; P = 0.03).
The prevalence of obesity has been gradually increasing worldwide during the past few decades. Obese persons are more likely to have hypertension, dyslipidaemia, and diabetes mellitus (DM) than non-obese persons, which are important causes of cardiovascular and cerebrovascular diseases. Accordingly, mortality rates are higher in this population [1]. It is also highly associated with the development of chronic kidney disease (CKD), microalbuminuria, and overt proteinuria [2]. With such increasing prevalence, many critically ill patients who require treatment in the intensive care unit (ICU) also have obesity. A previous meta-analysis from the United States showed that approximately 30% of ICU patients had a body mass index (BMI) of = 30 kg/m2, and these patients had prolonged durations of mechanical ventilation and lengths of ICU stay [3].
Acute kidney injury (AKI) occurs in many hospitalized and ICU patients, and is significantly associated with adverse outcomes such as high mortality, increased length of hospital stay, and progression to CKD [4]. Given the high burden of comorbidities accompanying obesity, it can be inferred that obese patients are prone to the development of AKI and thus experience more serious complications than non-obese patients with critical conditions. However, this assumption has not yet been clearly proven, and there has been much controversy on the relationship between AKI, obesity, and mortality. In fact, several studies have shown that obesity is associated with a high incidence and severity of AKI [5–7], as well as increased mortality in ICU patients [6]. In contrast, an inverse or null relationship between obesity and mortality has also been reported in other studies [5,8–12].
This phenomenon, called the ‘obesity paradox’, has been observed in patients with chronic diseases. In particular, in CKD patients, a higher BMI is associated with a lower risk of all-cause mortality [13], than a normal or lower BMI. Furthermore, recent evidence has shown that obese patients undergoing ICU and ventilator therapy have a survival benefit over non-obese patients [5]. Although the physiologic mechanism is not yet established, fat tissue as an energy reservoir has been suggested to explain this beneficial effect of obesity [14]. Of note, sepsis is characterized by increased protein catabolism and high energy consumption. It can be presumed that this process can be more deteriorated particularly when complicated by AKI in patients requiring renal replacement therapy. Therefore, we aimed to delineate the relationship between obesity and mortality in critically ill patients with AKI who were treated with continuous renal replacement therapy (CRRT).
We conducted an observational study in 212 adult patients who were treated with CRRT in the ICU of our institution between January 2010 and December 2014. A total of 573 patients were initially assessed for study eligibility. Patients were excluded if they were ≤ 18 or ≥ 75 years old, had end-stage renal disease (ESRD) on dialysis, or had stage 4 malignancy. Patients who had no BMI data were also excluded (Fig. 1). The study was approved by the Institutional Review Board of Yonsei University Severance Hospital (4-2010-0440). Since current study was a retrospective observational study and the study subjected de-identified, the IRB waived the need for written consent from the patients.
Demographic factors and comorbid conditions were obtained from electronic medical records. The Charlson comorbidity index (CCI) was used to evaluate the severity of the patients’ comorbidities [15], and BMI was calculated by using the height and weight data obtained on ICU admission (weight [kg]/height [m2]). The causes of AKI were classified into five categories: (i) sepsis, (ii) nephrotoxin, (iii) hypovolemic ischemia, (iv) surgery, and (v) others. Blood samples were collected immediately after ICU admission. The measured laboratory data included white blood cell (WBC) count, haemoglobin, haematocrit, platelet, prothrombin time, partial thromboplastin time, cholesterol, albumin, blood urea nitrogen, and creatinine. The estimated glomerular filtration rate (eGFR) was determined by using the Modification of Diet in Renal Disease equation [16]. The average vital signs, PaO2, FiO2, and Glasgow coma scale score in the first 24 hours of ICU admission were collected to calculate the sepsis-related organ failure assessment (SOFA) score.
The primary outcome was death from any cause that occurred at 30 days after CRRT initiation. Secondary outcomes included death from any cause occurred at 60 and 90 days after CRRT initiation, and weaning from CRRT due to renal recovery.
Continuous variables were expressed as mean ± standard deviation, and compared with t-test and one-way ANOVA. The normality of the distribution of parameters was analysed by using the Kolmogorov-Smirnov test. If data did not show a normal distribution, these were presented as median and interquartile range and compared by using the Mann-Whitney test or Kruskal-Wallis test. Categorical variables were expressed as percentages and compared with the chi-square test. Cumulative survival curves were derived using the Kaplan-Meier method, and differences between curves were analysed by log-rank test. To evaluate the relationship of BMI, covariables, and mortality, a Cox-proportional hazard model was used, and the results were presented as a hazard ratio (HR) and 95% confidence interval (CI). In addition, receiver operating characteristic (ROC) curve analysis was conducted and the cut-off value of BMI for the outcome was derived by Youden index, which is the maximum vertical distance between ROC curve and chance line. Statistical significance was defined as P < 0.05. All analyses were conducted by using IBM SPSS Statistics, version 20.0 (IBM Co., Armonk, NY, USA) and R language (version 3.3.1; R Foundation for Statistical Computing, Vienna, Austria).
The baseline characteristics of the patients and the CRRT prescription are presented in Tables 1 and 2, respectively. We classified patients into tertiles according to BMI: Q1, 13.5–21.8 kg/m2; Q2, 21.9–25.4 kg/m2; and Q3, 25.5–37.1 kg/m2. The average BMI was 23.9 ± 4.3 kg/m2. The mean age was 62.1 years, and 74 patients (34.9%) were women. The prevalence of hypertension, DM, and other comorbidities did not significantly differ between groups. The mean age-adjusted CCI score was 4.6 ± 2.6 and was similar in the three groups. Sepsis was a predominant cause of AKI (77.8%), and occurred less frequently in high tertiles; however, the difference did not reach statistical significance (P = 0.3). The mean eGFR at the time of starting CRRT was 26.3 ± 22.3 mL·min−1·1.73 m−2, and the initial kidney function of lowest tertile was significantly higher than those of other two quartiles (P = 0.008). The average SOFA score was 14.2 ± 3.1, and the severity of illness was not significantly different between groups (P = 0.54).
The ICU and hospital stay durations, survival days, and 30, 60 and 90-day mortality rates are presented in Table 2. The mean ICU and hospital stay was 6 (3–15) and 11 (3–32) days, respectively. Patients with a higher BMI stayed longer in the ICU and hospital than those with a lower BMI; however, the differences did not reach statistical significance. A total of 141 deaths (66.5%) occurred during 30 days after CRRT initiation. Most patients died of sepsis and there was no difference in cause of death between groups (data not shown). Thiry-nine patients (57.4%) in the highest tertile died as compared with 58 patients (78.4%) in the lowest tertile (P = 0.02). The Kaplan-Meier curve of the 30-day shows that the cumulative survival of the highest BMI tertile group was significantly higher than those of the lowest BMI group (Fig. 2, P = 0.04). An additional 19 deaths occurred between 30 and 90 days. When we analysed the 90-day mortality rate, there were 49 deaths (72.1%) in the highest tertile as compared with 60 deaths (81.1%) in the lowest tertile (P = 0.37). There were no differences of renal survival at all time points.
We further analysed the association between BMI and mortality by using multivariable-adjusted Cox models. To this end, we constructed four different models (Table 3). In model 1, in which sex, age-adjusted CCI score, septic AKI, and SOFA score were entered, the highest tertile of BMI was significantly associated with a decreased risk of death (HR, 0.58; 95% CI, 0.38–0.88; P = 0.01). In model 2, we additionally adjusted WBC and albumin as inflammatory and nutritional markers, and found that the survival benefit of a high BMI persisted. Finally, in model 3, CRRT prescription was added to model 2. In this fully adjusted model, patients in the highest tertile had a significantly decreased risk of death compared with those in the lowest tertile (HR, 0.57; 95% CI, 0.37–0.87; P = 0.01). When BMI was analysed as a continuous variable, a high BMI was still independently associated with a decreased risk of death (HR, 0.94 per 1 kg/m2 increase; 95% CI, 0.90– 0.99; P = 0.007). We further analysed the 60-day and 90-day mortality in these patients, and obtained similar results. In subgroup analyses according to presence of sepsis and cancer, survival benefit of high BMI was seen in only patients with sepsis (Fig. 3). In addition, to evaluate the cut-off value of BMI as a prognostic marker for outcome, ROC analysis was conducted. The area under ROC curve for BMI was 0.616, and optimal point of BMI derived from Youden index was 22.9 kg/m2.
In this study, we showed that a higher BMI is associated inversely with mortality in AKI patients undergoing CRRT. By using different multivariable-adjusted models, we found a consistent survival advantage of a higher BMI over a lower BMI, even though patients with lower BMI had a superior initial kidney function than patients with higher BMI. With ROC curve, we showed that patients with BMI over 22.9 kg/m2, which is defined as overweight and obesity in Asian population by World Health Organization criteria, had low risk of mortality. Thus, our findings are robust and can add evidence to the recently prevailing concept of the ‘obesity paradox’, even in critically ill patients undergoing renal replacement therapy.
We particularly paid attention to AKI patients treated with CRRT. Critical ill patients are generally hypercatabolic, and have substantial energy expenditure in proportion to the amount of stress [17]. In addition, patients with AKI have a high prevalence of malnutrition [18], and protein can be excessively degraded in uraemia caused by AKI [19]. Especially, loss of protein can be accelerated in AKI with CRRT, since protein and other nutritional elements are lost through CRRT [20]. Previous studies showed that 17% of centrally infused protein losses into CRRT effluent [21]. Accordingly, the American Society for Parenteral and Enteral Nutrition/Society of Critical Care Medicine Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient recommend that patients receiving hemodialysis or CRRT should receive increased protein, up to a maximum of 2.5 g/kg/day, and protein should not be restricted in patients with renal insufficiency [22]. In this regard, AKI patients on CRRT are more susceptible to a loss of energy reserve. To our knowledge, few studies have examined the relationship between obesity and adverse outcome in this exceptionally highly catabolic group. Considering this background, we sought to investigate whether obesity can exert protective effects against continuously energy-depleting conditions, and found that the obesity paradox indeed exists in these patients.
In general, obesity portends a high risk of developing adverse cardiovascular outcomes. However, contrary to this concept, many epidemiologic studies revealed an inverse relationship between obesity and mortality not only in chronic diseases [23,24] but also in the general population [25]. Recent evidence also indicates that this opposite relationship may hold true for acutely and seriously ill patients under various conditions [5,7,10,26]. In fact, obesity has been considered a significant predictor of AKI. A recent observational cohort study also found that obese patients were at high risk of developing AKI than patients with normal BMI [6]. Conversely, obesity plays a different role in acutely ill patients, even in those having AKI. A previous meta-analyses involving a large number of ICU patients have suggested that patients with a higher BMI are more likely to survive than those with a lower BMI [3,9,11,27]. However, these studies require cautious interpretation because their meta-analyses were statistically heterogeneous and detailed adjustments were not made. Our study has the merit of applying three different multivariable models, taking all potential factors into account. Our database included demographic and laboratory data, comorbidity index, vital sign monitoring, scoring system for the severity of illness, and CRRT prescription. Thus, we adjusted all these factors and found an inverse relationship between BMI and mortality, in agreement with previous studies [5,7–11].
A possible mechanistic link between obesity and a low death rate is largely presumptive. It is generally accepted that fat tissue can function as an energy reservoir; thus, obese patients can tolerate stressful and damaging conditions better than non-obese patients [28]. A recent study by Robinson et al [29] investigated the relationship among obesity, nutritional status, and mortality. In accordance with our study, they showed that high BMI was significantly associated with survival benefit in critical ill patients. Of note, they found malnutrition is less prevalent in obese patients than in underweight and normal patients, suggesting nutrition as a potential factor to explain survival advantage of obesity. Short-term protective cytokine profiles in obese patients [30,31] and other protective effects conferred by higher muscle mass [32] and fat tissue [33] have also been suggested as possible mechanisms responsible for this phenomenon. In aggregates, it can be presumed that obesity provides substantial energy resources and protective effects, and negates the harmful effects caused by inflammation, infection, and cardiovascular events.
Experimental evidence can also explain the favourable effects of obesity in AKI animal models. In a study by Sleeman et al [34], AKI was prevented by feeding a high-fat diet to adult swine that underwent cardiopulmonary bypass. The authors suggested a ‘pre-conditioning’ effect of obesity against abrupt bursts of hyper-inflammation, which can attenuate pro-inflammatory redox signalling and help maintain renal vascular and tubular homeostasis. Altered adipokine and cytokine profiles from adipose tissue can also play a role. Adiponectin and tumour necrosis factor-α receptors are produced by adipose tissue and can exert protective effects by decreasing inflammation [31].
Several shortcomings should be discussed. First, although we created multivariable models adjusted for many potential factors, this is an observational study with a relatively small sample size. Hence, unknown bias cannot be entirely excluded and our findings need to be interpreted with caution. Second, we used only BMI to evaluate obesity. BMI is easily measured and is the most widely used measure of obesity. However, it does not accurately reflect body composition [35]; thus, BMI is limited in assessing obesity. Other parameters such as abdominal diameter can be added to increase the diagnostic accuracy for obesity. Third, WBC and serum albumin were entered as inflammatory and nutritional markers in this study. Our database had laboratory data for CRP, a better marker of inflammation. However, > 30% of the data were missing and thus cannot be used for analysis. In addition, serum albumin is a well-known nutritional marker [36]; however, it has a limited ability in reflecting nutritional status in critically ill patients because it is also a negative acute-phase reactant [37]. Nevertheless, many studies have shown that high WBC and low serum albumin level are independent predictors of mortality in critically ill patients [38,39]. For this reason, these two parameters were adjusted in a multivariable Cox model. Finally, area under ROC curve for BMI was less than 0.7 in our analysis, suggesting that BMI is not a powerful predictor of mortality. Our study had relatively small sample size and thus further large-scale investigations are needed to verify BMI as a useful predictor of mortality of AKI patients with CRRT.
In conclusion, this study showed that high BMI is associated with survival benefit in AKI patients undergoing CRRT. Although the causality is uncertain, our findings suggest that obesity can function as an energy reservoir in this continuously energy-depleting condition.
Figure 1
Flowchart of participants in the cohort
AKI, acute kidney injury; BMI, body mass index; ESRD, end stage renal disease.
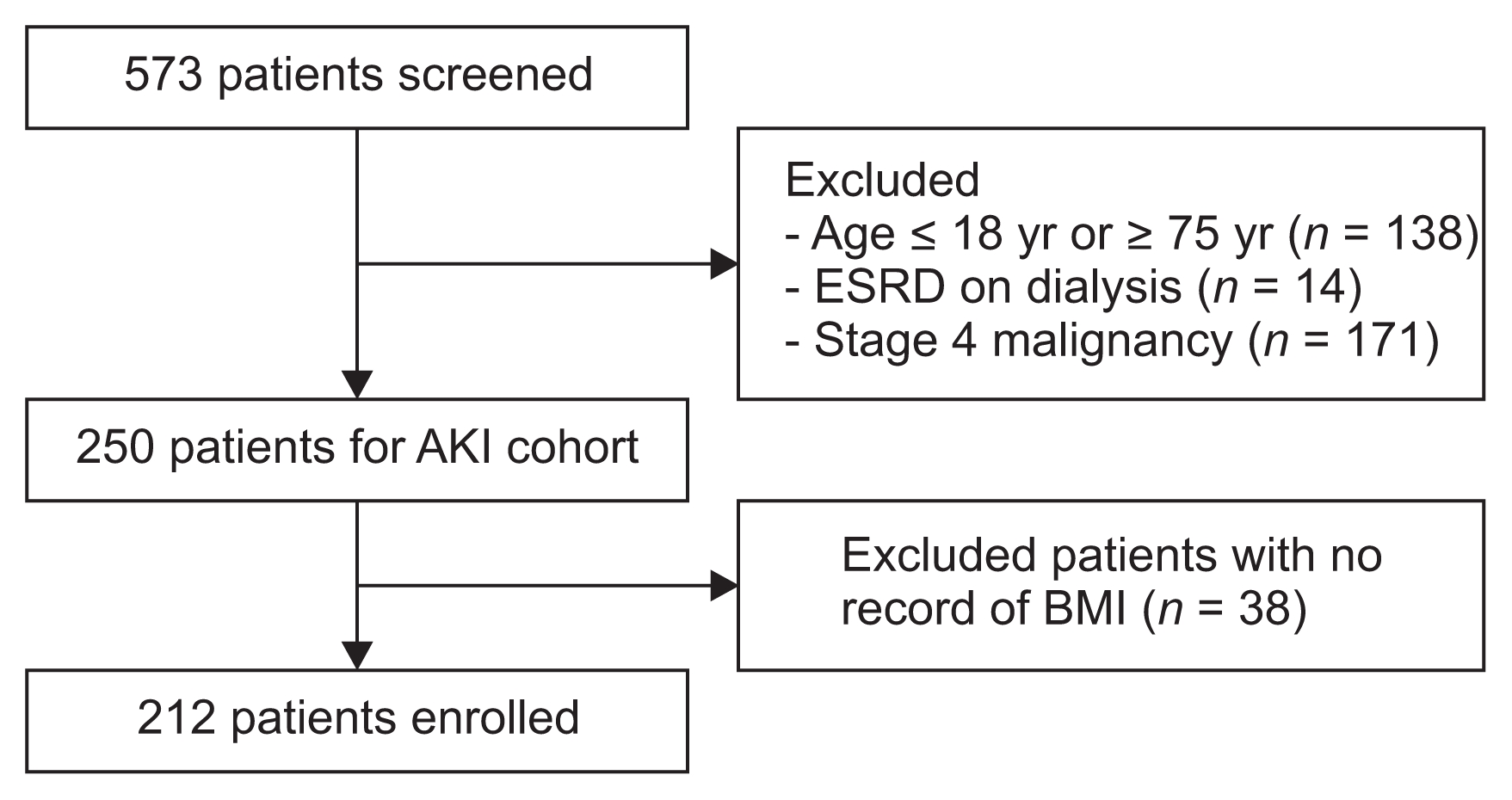
Figure 3
Hazard ratio for mortality according to presence of sepsis and cancer in fully adjusted model
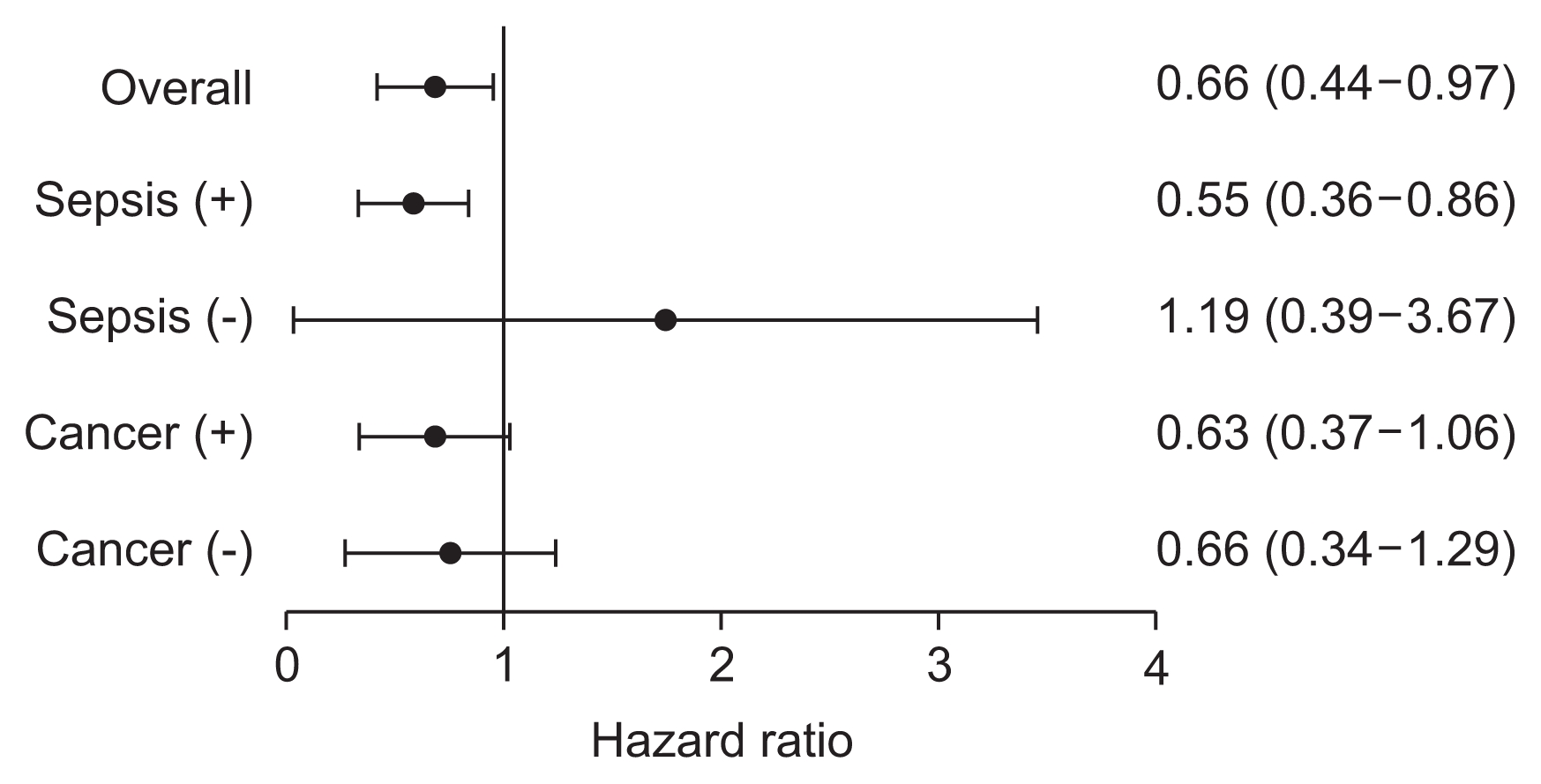
Table 1
Baseline characteristics of patients according to BMI tertiles
Table 2
Length of stay, survival, and mortality according to body mass index tertiles
Table 3
Multivariable Cox regression analyses for mortality
References
1. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 309:71–82. 2013;



2. Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 6:2364–2373. 2011;



3. Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med 36:151–158. 2008;


4. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16:3365–3370. 2005;


5. Soto GJ, Frank AJ, Christiani DC, Gong MN. Body mass index and acute kidney injury in the acute respiratory distress syndrome. Crit Care Med 40:2601–2608. 2012;



6. Danziger J, Chen KP, Lee J, Feng M, Mark RG, Celi LA, Mukamal KJ. Obesity, acute kidney injury, and mortality in critical illness. Crit Care Med 44:328–334. 2016;



7. Druml W, Metnitz B, Schaden E, Bauer P, Metnitz PG. Impact of body mass on incidence and prognosis of acute kidney injury requiring renal replacement therapy. Intensive Care Med 36:1221–1228. 2010;


8. Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest 123:1202–1207. 2003;


9. Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity (Silver Spring) 16:515–521. 2008;


10. Chao CT, Wu VC, Tsai HB, Wu CH, Lin YF, Wu KD, Ko WJ. NSARF Group. Impact of body mass on outcomes of geriatric postoperative acute kidney injury patients. Shock 41:400–405. 2014;


11. Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG, Peek N. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med 41:1878–1883. 2013;


12. Arabi YM, Dara SI, Tamim HM, Rishu AH, Bouchama A, Khedr MK, Feinstein D, Parrillo JE, Wood KE, Keenan SP, Zanotti S, Martinka G, Kumar A, Kumar A. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: an international multicenter cohort study. Crit Care 17:R722013;



13. Navaneethan SD, Schold JD, Kirwan JP, Arrigain S, Jolly SE, Poggio ED, Beddhu S, Nally JV Jr. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol 8:945–952. 2013;



14. Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, Kovesdy CP, Kalantar-Zadeh K. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 56:415–425. 2014;


15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. 1987;


16. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470. 1999;


17. Hwang TL, Huang SL, Chen MF. The use of indirect calorimetry in critically ill patients--the relationship of measured energy expenditure to Injury Severity Score, Septic Severity Score, and APACHE II Score. J Trauma 34:247–251. 1993;


18. Fiaccadori E, Lombardi M, Leonardi S, Rotelli CF, Tortorella G, Borghetti A. Prevalence and clinical outcome associated with preexisting malnutrition in acute renal failure: a prospective cohort study. J Am Soc Nephrol 10:581–593. 1999;


19. Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73:391–398. 2008;


20. Druml W. Metabolic aspects of continuous renal replacement therapies. Kidney Int Suppl (72):S56–S61. 1999;

21. Scheinkestel CD, Adams F, Mahony L, Bailey M, Davies AR, Nyulasi I, Tuxen DV. Impact of increasing parenteral protein loads on amino acid levels and balance in critically ill anuric patients on continuous renal replacement therapy. Nutrition 19:733–740. 2003;


22. McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 33:277–316. 2009;


23. Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Li W, Johnson J, Heymsfield SB, Cefalu WT, Ryan DH, Hu G. Body mass index and the risk of all-cause mortality among patients with type 2 diabetes mellitus. Circulation 130:2143–2151. 2014;



24. Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes. JAMA 308:581–590. 2012;



25. Veronese N, Cereda E, Solmi M, Fowler SA, Manzato E, Maggi S, Manu P, Abe E, Hayashi K, Allard JP, Arendt BM, Beck A, Chan M, Audrey YJ, Lin WY, Hsu HS, Lin CC, Diekmann R, Kimyagarov S, Miller M, Cameron ID, Pitkälä KH, Lee J, Woo J, Nakamura K, Smiley D, Umpierrez G, Rondanelli M, Sund-Levander M, Valentini L, Schindler K, Törmä J, Volpato S, Zuliani G, Wong M, Lok K, Kane JM, Sergi G, Correll CU. Inverse relationship between body mass index and mortality in older nursing home residents: a meta-analysis of 19,538 elderly subjects. Obes Rev 16:1001–1015. 2015;


26. Bundhun PK, Li N, Chen MH. Does an obesity paradox really exist after cardiovascular intervention?: a systematic review and meta-analysis of randomized controlled trials and observational studies. Medicine (Baltimore) 94:e19102015;


27. Hogue CW Jr, Stearns JD, Colantuoni E, Robinson KA, Stierer T, Mitter N, Pronovost PJ, Needham DM. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med 35:1152–1170. 2009;


28. Kee AL, Isenring E, Hickman I, Vivanti A. Resting energy expenditure of morbidly obese patients using indirect calorimetry: a systematic review. Obes Rev 13:753–765. 2012;


29. Robinson MK, Mogensen KM, Casey JD, McKane CK, Moromizato T, Rawn JD, Christopher KB. The relationship among obesity, nutritional status, and mortality in the critically ill. Crit Care 43:87–100. 2015;

30. Stenvinkel P, Marchlewska A, Pecoits-Filho R, Heimbürger O, Zhang Z, Hoff C, Holmes C, Axelsson J, Arvidsson S, Schalling M, Barany P, Lindholm B, Nordfors L. Adiponectin in renal disease: relationship to phenotype and genetic variation in the gene encoding adiponectin. Kidney Int 65:274–281. 2004;


31. Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol 277:E971–E975. 1999;


32. Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 14:2366–2372. 2003;


33. Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, Block G, Kopple JD. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr 83:202–210. 2006;


34. Sleeman P, Patel NN, Lin H, Walkden GJ, Ray P, Welsh GI, Satchell SC, Murphy GJ. High fat feeding promotes obesity and renal inflammation and protects against post cardiopulmonary bypass acute kidney injury in swine. Crit Care 17:R2622013;



35. Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 32:959–966. 2008;



36. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med 128:1023.e1–e22. 2015;


37. Fleck A. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc Nutr Soc 48:347–354. 1989;






 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















