Introduction
The highly-conserved exocyst complex comprises eight proteins, Exoc1ŌĆō8 (originally named Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84). The exocyst was first identified in the budding yeast, Saccharomyces cerevisiae. The six Sec proteins, named because mutations inhibited secretion (or exocytosis), were discovered by Novick, Field, and Schekman almost four decades ago in their classic genetic screen. These investigators had a simple, yet elegant idea, that if the mother cell could not secrete into the daughter cell, then S. cerevisiae mutant cells that were temperature-sensitive for secretion and cell surface growth would become heavier during incubation at the non-permissive temperature (37┬░C). This would allow for the selection of mutants by sedimentation of mutagenized cells on a Ludox density gradient [1]. This work was at least partially responsible for Dr. Randy Schekman being awarded the Nobel Prize in Physiology or Medicine in 2013. TerBush and Novick [2] later purified the exocyst protein complex containing Exoc1-6 (the six Sec proteins) and two additional subunits, Exoc7 and Exoc8 (Exo70 and Exo84, respectively). Mammalian homologues of all eight yeast exocyst proteins were identified from rat brains by Hsu et al [3] in 1996. All of the exocyst components are hydrophilic proteins which mutually interact to form a 19.5S complex peripherally associated with the plasma membrane [4]. The exocyst is thought to act as a holocomplex.
Regulation of the exocyst complex
In yeast, mutants of individual exocyst proteins accumulate vesicles in the cells because the vesicles cannot dock or fuse with the plasma membrane. The exocyst proteins localize to regions of active cell surface expansion, the bud tip at the beginning of the cell cycle and the mother-daughter cell connection during cytokinesis. Therefore, the exocyst complex is considered involved in directing vesicles to their precise sites of fusion [5ŌĆō8].
The exocyst plays a central role in exocytosis; however, how the exocyst is regulated remains unclear. The exocyst, due to its complexity and ubiquity, potentially integrates many different inputs. Recent data from our laboratory and other studies have shown multiple small GTPases regulate the exocyst, including members of the Rab [6], Rho [9ŌĆō14], Ral [15ŌĆō18], and Arf [19,20] families (Table 1). Guanine nucleotide exchange factors (GEFs) of the small GTPases, such as Tuba, a GEF for Cdc42 [14,21], and Sec2 (mammalian homolog is Rabin8), a GEF for Sec4 (mammalian homolog is Rab8) [22], have also been shown to regulate exocyst (Table 1).
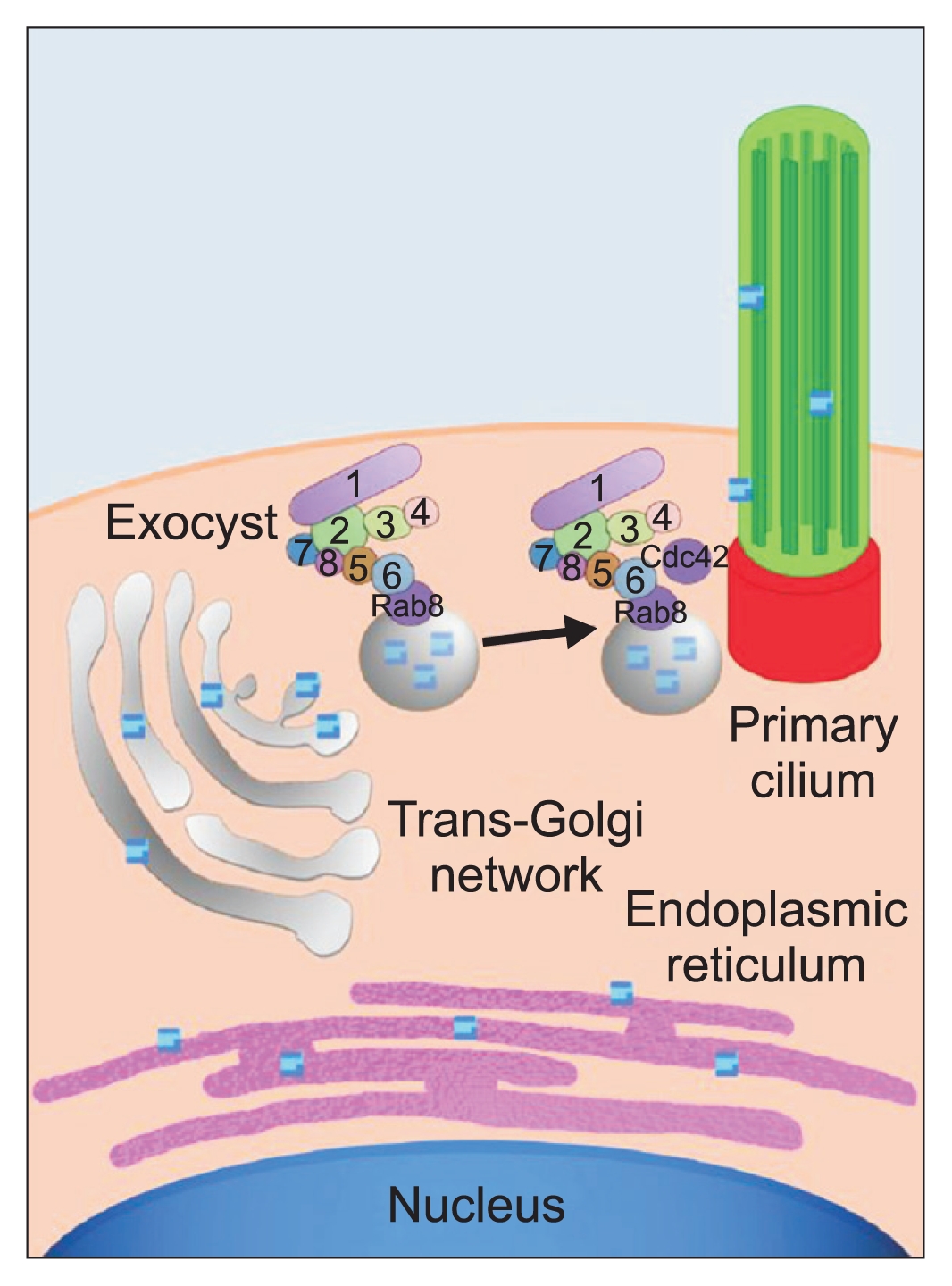
The first small GTPase found to interact with the exocyst complex was Sec4 (mammalian homolog Rab8), the founding member of the Rab family (Fig. 1) [6]. Rabs are important regulators of all vesicular trafficking events and Sec4 is essential for a post-Golgi event in yeast secretion. Genetic analysis indicated Sec4 functions upstream of the exocyst. The exocyst component Exoc6, specifically associates with secretory vesicles and interacts with Sec4-GTP, which is found on the surface of the vesicular membrane. The interaction of Sec4-GTP with Exoc6 triggers further interactions between Exoc6 and other exocyst components, eventually leading to docking and fusion of vesicles with specific domains in the plasma membrane.
Because the exocyst localizes specifically to regions of active secretion and cell growth, how this localization is controlled is important. The second class of GTPases found to interact with the exocyst are members of the Rho family. Several rho1 mutant alleles were identified in a search for mutants regulating the localization of green fluorescent protein (GFP)-tagged exocyst subunits in budding yeast [11]. The best-known Rho family function is organizing the actin cytoskeleton; however, Rho1 exerts different effects on the exocyst via direct interaction between Rho1-GTP and the exocyst component Exoc1, which has been proposed as a ŌĆ£landmarkŌĆØ for defining polarized domains in the plasma membrane [5]. Another Rho family protein, Cdc42, was also shown to interact with Exoc1 and was required for the initial targeting of Exoc1 to the emerging yeast bud. Both Rho1 and Cdc42 interact with the Exoc1 N-terminus and mutually compete for Exoc1 binding in vitro. Possibly, Cdc42 and Rho1 interact with Exoc1 at different stages of the yeast cell cycle in vivo [13].
In addition to the direct interaction of Rho1 and Cdc42 with Exoc1, another Rho family protein, Rho3, was shown to associate with Exoc7, a different exocyst subunit [9,12]. The interaction between Rho3 and Exoc7 was greatly reduced when mutations were introduced into the Rho3 effector domain. The interaction between Rho3 and Exoc7 was dependent on the presence of Rho3-GTP [12]. Mutations in the Rho3 effector domain revealed roles in the regulation of actin organization, transport of exocytic vesicles to the bud, and docking of vesicles with the plasma membrane. Reportedly, Exoc7 mediates vesicle docking [9].
The mammalian exocyst (Sec6/8 complex) also localizes to areas of active exocytosis. In developing neurons, the exocyst localizes to growth cones and the tips of growing neurites [23]. In renal epithelial cells, the exocyst is concentrated near the tight junctions, a postulated region of active basolateral membrane addition [4], and the primary cilium [24], and regulates transport of vesicles between the trans-Golgi network (TGN) and plasma membrane [25]. When cultured in three-dimensional (3D) collagen gels, Madin-Darby canine kidney (MDCK) epithelial cells form multicellular cysts and, in response to hepatocyte growth factor, form tubules. This in-vitro system is a good model for studying the molecular pathways by which epithelial cells form these higher order structures [26,27]. Using this system, we showed the exocyst complex relocalized concomitant with changes in cell polarity that occur during these processes. Moreover, overexpression of the exocyst subunit Exoc5 specifically increased the synthesis of secretory and basolateral proteins, as well as the formation of cysts and tubules, indicating the exocyst is centrally involved in these higher order processes [28].
The exocyst is centrally involved in ciliogenesis
As stated above, the exocyst is involved in multiple cellular processes including basolateral transport [4,28], ciliogenesis [29], and protein translation in the endoplasmic reticulum [30,31]. Therefore, determining the mechanism by which the exocyst is involved in these processes is important. The involvement of the exocyst in primary ciliogenesis is particulary important because primary cilia are centrally involved in the pathogenesis of autosomal dominant polycystic kidney disease (ADPKD), the most common, potentially lethal genetic disease in humans, affecting 12,000,000 patients worldwide. In ADPKD, massive cystogenesis mechanically destroys the kidneys [32].
In 2017, the crystal structure of the central exocyst component, EXOC5, was solved [33], and an in-vivo 3D integrative approach to the exocyst was performed [34]. The details of the exocyst complex structure using cryoelecron microscopy was recently reported [35]. EXOC5 contains a VxPx ciliary targeting sequence that is highly conserved from yeast to humans. We analyzed solvent accessibility of the Val666, Ala667, and Pro668 residues of EXOC5 represented by the 5h11 structure [33]. Solvent-accessible surface areas were 37, 49, and 52 ├ģ2 and relative accessibilities were 32%, 73%, and 50%, respectively. Thus, all three residues are exposed to solvent and available for binding, although proline to a greater degree than valine. Therefore, we performed site-directed mutagenesis of EXOC5-myc cDNA in a pcDNA3 vector, mutating the cytosine at position 2002 to a guanine (cca to gca), causing translation of alanine instead of proline. Successful site-directed mutagenesis was confirmed by sequencing the full cDNA transcript. The pcDNA3 vector containing the mutated human EXOC5-myc ciliary targeting sequence was transfected into MDCK cells and stable cell lines generated. Three clonal cell lines expressing the mutated human EXOC5-myc ciliary targeting sequence were identified (G5, G7, and G9) using an antibody we generated against human EXOC5 [29].
The EXOC5 ciliary targeting sequence-mutated protein was confirmed stable and able to bind other members of the exocyst complex. Next, stably controlled, EXOC5-overexpressing (OE), Exoc5 knockdown (KD), and EXOC5 ciliary targeting sequence-mutated MDCK cells, were cultured on Transwell filters. Results showed primary ciliogenesis was increased in EXOC5 OE cells and inhibited in Exoc5 KD and EXOC5 ciliary targeting sequence-mutated cells. Next, the EXOC5 OE, Exoc5 KD, EXOC5 ciliary targeting sequence-mutated, and control MDCK cells, were grown in collagen gels until the cyst stage. Results showed EXOC5 OE cells formed mature cysts with single lumens more rapidly than control cysts, and Exoc5 KD and EXOC5 ciliary targeting sequence-mutated MDCK cells failed to form mature cysts, indicating the exocyst, acting through the primary cilium, is necessary for cystogenesis. Hepatocyte growth factor was added to induce tubulogenesis in the EXOC5 OE, Exoc5 KD, EXOC5 ciliary targeting sequence-mutated, and control cell cysts. EXOC5 OE cell cysts formed tubules more efficiently than control MDCK cell cysts. EXOC5 ciliary targeting sequence-mutated MDCK cell cysts formed significantly fewer tubules than control cell cysts, and Exoc5 KD cysts did not undergo tubulogenesis, indicating the exocyst, acting through the primary cilium, is necessary for renal tubulogenesis. Finally, EXOC5 messenger RNA (mRNA) completely rescued the ciliary phenotypes in exoc5 mutant zebrafish, and the EXOC5 ciliary targeting sequence-mutated mRNA could no longer efficiently rescue the phenotypes. Taken together, these data showed the exocyst, acting through the primary cilia, was necessary for renal ciliogenesis, cystogenesis, tubulogenesis, and development (Fig. 1) [36].
Exoc5 floxed mice
Because Exoc4 global knockout mice die very early during embryogenesis (during gastrulation) [37], a floxed exocyst mouse line was generated to study the function of the exocyst in murine kidneys. To the best of our knowledge, in 2015 we generated the first and only exocyst floxed mouse, Exoc5fl/fl [38]. Kidney-specific Exoc5 knockout mice that survived for 30 days had cystic kidney disease [39].
The exocyst is involved in other renal processes and diseases
We hypothesized that different small GTPases found at different locations in the cell, give the exocyst specificity of function. Using cell culture, zebrafish, and kidney-specific knockout mice, Cdc42, a Rho family member, was found at the primary cilium and shown to regulate the exocyst [10]. Similarily, Tuba, a ciliary Cdc42 GEF, regulates the exocyst and is necessary for proper ciliogenesis, cystogenesis, and tubulogenesis [14,21]. Arl13b, an Arf family member, in its GTP form was shown to regulate the exocyst. Arl13b and Cdc42 genetically interact in zebrafish, and knockout of Arl13b in mice leads to renal cystogenesis, which phenocopies mice surviving for 30 days after kidney-specific knockout of Exoc5 [20]. Because multiple small GTPases appear to regulate the exocyst at the primary cilium, the exocyst, in addition to trafficking vesicles to the primary cilium, may have other function(s) in the primary cilium (e.g., secretion or retrieval of small extracellular vesicles). We have previously shown the exocyst, as well as regulators of the exocyst, are found in human urinary extracellular vesicles [40].
Another function of the exocyst in the kidney may be a role in the injury and/or recovery of renal tubule epithelial cells. Exoc5 overexpression in MDCK cells protects the cells from injury, which is mediated through the MAPK pathway [41ŌĆō43]. This may involve primary cilia because unilateral nephrectomy in mice elongates primary cilia in the remaining kidney [44]. We are currently generating proximal-tubule specific Exoc5 OE and knockout mice to test this hypothesis. Preliminary results show that proximal tubule-specific Exoc5 knockout mice are significantly more susceptible to ischemia/reperfusion injury than control littermate mice (unpublished data).
In addition, we examined the role of the exocyst in podocyte development and disease. We identified two patients with exocyst deletions. In addition, all podocyte-specific Exoc5 knockout mice died with 30 days and exhibited severe proteinuria and renal failure. This was a cilia-independent exocyst effect, as podocyte-specific intraflagellar protein 88 (Ift88) knockout mice did not have proteinuria or a phenotype [45].
The exocyst in other organs
Due to the ubiquitous expression of the exocyst complex, we hypothesized the exocyst affects ciliogenesis in other organs. After examining exoc5 knockout zebrafish [46], and generating photoreceptor-specific Exoc5 knockout mice (photoreceptors are modified primary cilia) [47], the exocyst, regulated by Cdc42, was shown to be necessary for eye development. Photoreceptor-specific knockout of Exoc5 in mice resulted in blindness [47]. Our collaborators in South Korea showed the exocyst also appeared necessary for cilia development in the ear [48]. Finally, patients with ADPKD have abnormal cardiac valves [49], especially biscuspid aortic valves (BAV) [50]. We recently showed that cardiac valves have cilia during development, but not in adulthood [51], and endocardial-specific Exoc5 knockout mice have BAV (unpublished data).
Summary
The exocyst is a very important complex that targets and docks vesicles translocating from the TGN to various sites in renal cells, including the primary cilium. This complex appears to have many functions in the kidney that are likely a result of different small regulatory GTPases acting on the exocyst. By manipulating the exocyst and/or its regulators, many renal diseases and possibly diseases affecting other organs, could be treated.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















