| Kidney Res Clin Pract > Volume 37(4); 2018 > Article |
|
Abstract
Background
The most common cause of acute kidney injury (AKI) in pregnancy is preeclampsia. Serum cystatin C (CysC) is a potential biomarker of early kidney damage as its levels are not disturbed by volume status changes in pregnancy, and serum CysC levels could serve as a replacement for conventionally used creatinine. In this study, we investigated the serum levels of CysC in severe preeclampsia cases and the associations between CysC levels and poor obstetric outcomes.
Methods
Our cohort included severe preeclampsia patients with a normal serum creatinine level. Creatinine was measured to calculate estimated glomerular filtration rate (eGFR) based on the Cockcroft and Gault, Modification of Diet in Renal Disease Study (MDRD), and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations, while CysC was measured to calculated eGFR based on a CysC-based equation. We then evaluated the correlations between serum CysC level, eGFR, and obstetric outcomes.
Results
Twenty-six patients were evaluated of which 38.5% delivered preterm and 30.8% had low-birth weight babies. Unlike creatinine-based eGFR and CysC-based eGFR, serum CysC demonstrate significant negative correlation with gestational age. Receiver operating characteristic curve analysis indicated that serum CysC is a potential biomarker of preterm delivery with a cut-off serum level of 1.48 mg/L with 80% sensitivity and 75% specificity.
Preeclampsia is a pregnancy-specific disorder that affects 4% of pregnancies and is routinely diagnosed when a pregnant woman presents with increased blood pressure and proteinuria [1]. Preeclampsia is one of the main causes of maternal, fetal, and neonatal mortality, especially in developing countries [2]. The acute clinical importance of preeclampsia lies in its relation to maternal and neonatal mortality and morbidity. Pregnant women with preeclampsia can experience severe complications such as eclampsia, HELLP syndrome, pulmonary edema, or kidney failure [3]. The disease is also related to fetal growth restriction and preterm delivery. An increase in blood pressure can develop into renal dysfunction, and worsening of renal function will result in salt and water retention, which further complicate the hypertensive state. This vicious cycle can ultimately lead to severe pre-eclampsia, followed by poor obstetric outcome. Children born to mothers with preeclampsia have an increased risk of bronchopulmonary dysplasia and cerebral palsy caused by preterm birth and being small for gestational age [4,5].
The most common causes of acute kidney injury (AKI) in pregnancy are preeclampsia and eclampsia, which can ultimately lead to an increase in both fetal and maternal mortality rates [6]. Many biomarkers are available for the detection of kidney damage, and early AKI detection may be a potential predictor of preeclampsia [7–11].
Chapman et al [12] documented early rises in glomerular filtration rate (GFR) and kidney blood flow by inulin and p-aminohippurate clearance in association with systemic and kidney vasodilation in a series of 10 pregnant women [12]. However, as inulin and p-aminohippurate levels are determined by invasive investigation, these biomarkers are not suitable for screening for AKI in the setting of preeclampsia. Serum creatinine, which is widely used as a marker of kidney damage, has been shown to be a poor biomarker of early AKI in pregnancy because of its low sensitivity [13]. Kidney Disease Improving Global Outcomes (KDIGO) specified that normal serum creatinine levels are 0.4 to 0.8 rather than 0.8 to 1.2 in pregnancy due to underestimation caused by dynamic volume status. There are also no available creatinine-based formulae to accurately calculate estimated GFR (eGFR) in pregnant patients using serum creatinine [14]. Several studies have also shown that serum creatinine is unreliable compared to inulin, even though serum creatinine is the gold standard for calculation of GFR. Kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin are other promising biomarkers of AKI. However, because the levels of these markers increase rapidly and normalize within 48 hours, they are not appropriate for use as screening biomarkers [15].
Cystatin C (CysC) has been studied intensively in pregnant women because it is independent of body weight, muscle mass, and other pregnancy-related changes [16]. CysC is a 13 kDa proteinase inhibitor from the cystatin superfamily of cysteine protease inhibitors that play important roles in intra-cellular catabolism of proteins and peptides. Some studies have shown that this biomarker is a better indicator of kidney function than creatinine [17,18], but there are no widespread recommendations for its use, even in pregnancy [19]. More intensive studies will have to be conducted before it can become a routinely used clinical biomarker. Several researches have described the utility of CysC as a biomarker in pregnancy. However, no prior study has investigated whether this biomarker is a predictor of poor obstetric outcomes [20–24].
In this study, we used serum creatinine and serum CysC to calculate eGFR using various formulae, and investigated if serum CysC level is associated with the obstetric complications of preterm delivery and low-weight babies in patients with severe preeclampsia.
We enrolled a prospective cohort of pregnant women diagnosed with severe preeclampsia who were receiving antenatal care at Her Royal Highness Princess Maha Chakri Sirindhorn Medical Center (MSMC), Thailand during August 1st, 2014 and September 30th, 2016. All women voluntarily signed a consent form before participating in our study. Data were collected from pregnant women with a maternal age more than 18 years and in the second half of pregnancy (gestational age, > 20 weeks). However, we included only severe preeclampsia cases with a gestational age of more than 28 weeks in our cohort. Those with a history or diagnosis of chronic hypertension, placenta previa, gestational diabetes, or chronic kidney disease with serum creatinine levels ≥ 0.8 mg/dL were excluded. Sample size was calculated using a power of 80%, alpha value of 0.05, and a dichotomous endpoint formula. Using data from a pilot project involving 10 pre-eclamptic patients (unpublished data), we found that the sensitivity and specificity of serum CysC for predicting preterm deliveries was 0.75 and 0.60, respectively. Using the Buderer approach and a severe preeclampsia prevalence of 1.3%, we calculated that we needed a minimum sample size of 25 [25,26].
A clinical study registration number for this study is TCTR20180730002. The protocol for patient participation was approved by the Human Research Ethics Board of Srinakharinwirot University (Issue #SWUEC/E-038/2557), and fulfilled the tenants of the 1964 Helsinki declaration and its amendments.
Severe preeclampsia was defined as a systolic blood pressure (SBP) ≥ 160 mmHg or a diastolic blood pressure (DBP) ≥ 110 mmHg on two occasions at least 4 hours apart in a previously normotensive patient with a positive urine protein result (at least 1+ of dipstick urine protein), impaired hepatic function, progressive renal insufficiency, or new onset cerebral/visual disturbance, pulmonary edema, or thrombocytopenia (platelet count, < 100,000/mm3) [27]. Preterm labor was defined as babies born alive before 37 weeks of pregnancy [28] and low birth weight as a weight at birth of less than 2,500 g (5.5 pounds) or a fetus in the lowest 10th percentile of the fetal growth chart (gestational age confirmed by both date and ultrasound in all patients) [29,30]. Teenage pregnancy was defined as births to mothers aged 15 to 19 years and elderly pregnancy was defined as birth by mothers older than 35 [31,32].
Patients with severe preeclampsia received a magnesium infusion based on MSMC guidelines if their gestational age was more than 28 weeks and the obstetrician had ensured lung maturity of the neonate. Within 24 hours after infusion of magnesium, termination was considered. All patients had blood samples taken for investigation of complete blood count, liver function tests, and creatinine and CysC level determination for initial admission before receiving a magnesium infusion. Clinical parameters such as blood pressure, volume of fluid intake, urine output, and blood loss were monitored routinely until end of delivery.
Serum creatinine was measured by the enzymatic method using a Cobas® 6000 analyzer (Roche Diagnostics, Mannheim, Germany). Serum CysC was analyzed by an immunoturbidimetric method using an ARCHITECT ci8200 instrument (Abbott Laboratories Ltd., Bangkok, Thailand). All formulae were adjusted for non-black female patients. The eGFR based on CysC was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) CysC equation (2012) [33]. The eGFR based on creatinine was calculated using the CKD-EPI creatinine equation (2009) [34], Modification of Diet in Renal Disease Study (MDRD) creatinine equation (2006) [35], and Cockcroft and Gault creatinine equation. Individual eGFR was assessed using a web-based calculator (https://www.kidney.org/professionals/kdoqi/gfr_calculator) [36] after entering serum creatinine or CysC, age, and standardization assay without body surface adjustment. The Cockcroft and Gault creatinine equation (eGFR-CG) [37] was expressed as follows:
Urine protein level was assessed semi-quantitatively using the colorimetric method of tetrabromophenol blue reagent in urine dipstick [38]. The threshold used to define a positive test result was 300 mg/24 hours, 300 mg/dL, or a dipstick proteinuria reading of more than 1+ defined based on the diagnostic criteria of preeclampsia [39]. Only patients diagnosed with preeclampsia based on hypertension and persistently high thresholds (> 1+ proteinuria) were included in the study.
IBM SPSS statistical software package ver. 23.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Continuous data were evaluated to determine whether they followed a normal distribution. Parametric data were evaluated using parametric tests and Pearson correlation coefficients. Non-parametric data were evaluated using non-parametric tests and Spearman’s coefficient was calculated to assess the associations between various parameters and obstetric outcomes. Mean values and standard deviations were compared among groups using Student’s t test. Pearson correlation and logistic regression analyses were used to determine the significance of correlations between the various clinical parameters and obstetric outcomes. The area under the receiver operating characteristic (ROC) curve was used to assess diagnostic accuracy and to perform cross-validation analysis. P values less than 0.05 were considered statistically significant.
Twenty-six pregnant women with severe preeclampsia who delivered at MSMC were analyzed in the present study. All patients had a normal serum creatinine level (0.40 to 0.80 mg/dL), and serum CysC ranged from 0.99 to 1.86 mg/L (1.43 ± 0.24 mg/L, mean ± standard deviation). Ten (38.5%) women who experienced preeclampsia were teenage or elderly pregnancies. The average gestational age at delivery was 37.07 ± 2.30 weeks. The incidence of preterm delivery was 38.5% (gestational age was confirmed by date and ultrasound). Incidence of low birth weight corrected by gestational age was 19.2% in this cohort (Table 1). Other complications often associated with preeclampsia like hepatitis or HELLP were not found in the present study. Other demographic data are presented in Table 1.
Four formulae were used to calculated eGFR: the CKD-EPI CysC equation (2012), CKD-EPI creatinine equation (2009), MDRD equation (2006), and Cockcroft and Gault equation. A significant directional correlation was found among all eGFR values. However, GFR estimated using the CysC-based calculation was drastically different from that estimated using the creatinine-based equations, with the CysC-based eGFR less than half of the creatinine-based eGFR values (Table 2, 3). Additionally, we found that up to 46.2% of severe pre-eclamptic patients had an eGFR < 60 mL/minute when calculated with CysC, whereas their serum creatinine levels were in the normal range. This implies that the creatinine-based equation may overestimate the real GFR, or that the CysC-based formula may underestimate the GFR. If the former is correct, CysC may potentially be more sensitive as an early AKI biomarker than creatinine.
Binary logistic regression analysis was employed to predict risk of the adverse obstetric outcomes of preterm delivery and low birth weight body adjusted for maternal age and systolic and DBP. Only serum CysC was a significant predictor of preterm delivery (odds ratio, 32.52; P < 0.01). The eGFR calculated by creatinine- or CysC-based formulae did not predict preterm delivery. Neither renal function biomarkers (creatinine and CysC) nor eGFR could predict a low birth weight (Table 4). Proteinuria (protein dipstick) and systolic and DBP were also not predictive of low birth weight or preterm delivery.
We further used ROC curve analysis to evaluate the accuracy of serum CysC for predicting preterm birth in our preeclampsia cohort. Analysis showed that the area under the curve was 0.738 with P = 0.045, indicating a high degree of discrimination.
ROC curve analysis suggested that the cut-off serum CysC level of > 1.48 mg/L was the best threshold for screening for the risk of preterm delivery in patients with preeclampsia with a sensitivity and specificity of 80% and 75%, respectively. Thus, a pregnant woman with in pre-eclampsia with a serum CysC level > 1.48 mg/L would be considered at risk for preterm delivery and require close monitoring and constant medical attention (Fig. 1).
Renal function is often closely monitored in pre-eclamptic patients as it is a major concern [40,41] with the primary goal of timely delivery before irreversible kidney injury develops. Serum creatinine, serum CysC, and uric acid concentration are parameters that have been used previously to monitor renal function in hypertensive disorders of pregnancy [42–45].
Hemodynamic adaptations in pregnant women is crucial for healthy pregnancy outcomes [46]. These adaptations include a reduction in vascular resistance, increase in cardiac output, and increase in intravascular volume via sodium and water retention [47]. These changes also affect other organs. For instance, there will be an increase in GFR and effective renal plasma flow within the kidney, which are essential for maintenance of electrolyte balance [48]. Poor pregnancy outcomes are often observed in pregnancies where these physiological changes have failed to occur [49]. A study by Piccoli et al [50] in 2015 demonstrated a worse prognosis in mothers with stage 1 CKD.
CysC is produced by nucleated cells at a constant rate, and is then filtered and excreted by nephrons. Despite early enthusiasm, however, CysC has not been shown to have obvious superiority compared to creatinine [51]. The reason for this is that even though CysC levels are relatively constant in individuals under normal circumstances, it can be affected by several factors including smoking, liver disease, thyroid disease, diabetes mellitus, extreme age, and obesity [52–57]. Additionally, the higher cost associated with measuring CysC and lack of availability of tests has hindered its acceptance as a creatinine replacement to estimate renal function, even in pregnant patients [58].
We found that GFR values estimated using both creatinine and CysC were directionally and proportionally correlated. However, CysC-based GFR was approximately half the value of the creatinine-based eGFR estimates, suggesting that CysC underestimates the true GFR value. This also indicates that there are uninvestigated components in the CysC formula that makes it unsuitable for accurate estimation of GFR in pregnant women. Interestingly, none of the eGFR values were correlated with a poor obstetric outcome.
CysC may nevertheless have prognostic importance. CysC, in addition to being a biomarker of AKI, is also a known marker of inflammation [58]. There is mounting evidence that CysC may be a predictor of adverse outcomes independent of renal function. A study by Koenig et al [59] examined the association between plasma CysC and risk of secondary cardiovascular (CV) events in a cohort of over a thousand individuals who had a history of coronary heart disease. Creatinine and estimated creatinine clearance were not significantly associated with risk of a cardiovascular event. In contrast, higher plasma CysC was associated with an increased risk of a CV event, even after adjusting for well-known risk factors, including C-reactive protein. Compared with individuals in the lowest quintile of CysC, those in the highest quintile had a more than a two-fold increase in risk, even after adjusting for estimated creatinine clearance [59]. In this study, we found a correlation between serum CysC level and preterm delivery. Serum creatinine, semi-quantitative urine protein level, and hypertension showed no ability to predict obstetric outcomes in patients with severe pre-eclampsia.
In normal pregnancy, serum CysC levels are high (0.89 ± 0.12 mg/L) in the first trimester, decrease significantly (0.65 ± 0.14 mg/L) during the second trimester (P < 0.001 compared to first trimester), and increase again (0.82 ± 0.19 mg/L) in the third trimester.
In this study, the average serum CysC levels during the third trimester in pre-eclamptic patients was 1.43 ± 0.24 mg/L (Table 1), which is significantly higher than in normal pregnancy. This suggests that CysC may have adequate sensitivity to detect abnormal renal function even when serum creatinine levels are within the normal range. This has clinical significance because even small reductions in renal function are associated with adverse pathophysiologic consequences. By plotting a ROC curve, we found that a serum CysC concentration of higher than 1.48 mg/L has a sensitivity of 80% and specificity of 75% to detect preterm delivery in patients with preeclampsia (Fig. 1).
In conclusion, although CysC is a biomarker for detection of early AKI, eGFR using CysC during pregnancy is unlikely to be accurate, suggesting unexplored inflammatory processes that cause preterm delivery in mothers with preeclampsia. Our results indicate that CysC is a valid inflammatory marker that can predict preterm delivery in addition to being a biomarker of AKI. Serum CysC level therefore has the potential to predict preterm delivery in patients with severe preeclampsia.
Acknowledgments
The authors would like to express our appreciation for a grant from the Her Royal Highness Princess Maha Chakri Sirindhorn Medical Center (MSMC), Thailand. We gratefully acknowledge use of the services and facilities of Her Royal Highness Princess Maha Chakri Sirindhorn Medical Center (MSMC), funded by MSMC Grant contract number 461/2557. We also would like to express our cordial thanks to the staff of the Department of Pathology, the Antenatal Care Unit, and the Labour Unit of the Department of Obstetrics and Gynecology, Faculty of Medicine, Srinakharinwirot University for clinical data review.
Figure 1
Receiver operating characteristic (ROC) curve of the predictive accuracy of cystatin C (CysC) for preterm delivery in preeclamptic patients.
The area under the ROC curve was 0.74, indicating a significant degree of discrimination (P = 0.04). CysC values > 1.48 mg/L were highly suggestive of preterm delivery in severe preeclamptic patients with 80% sensitivity and 75% specificity.
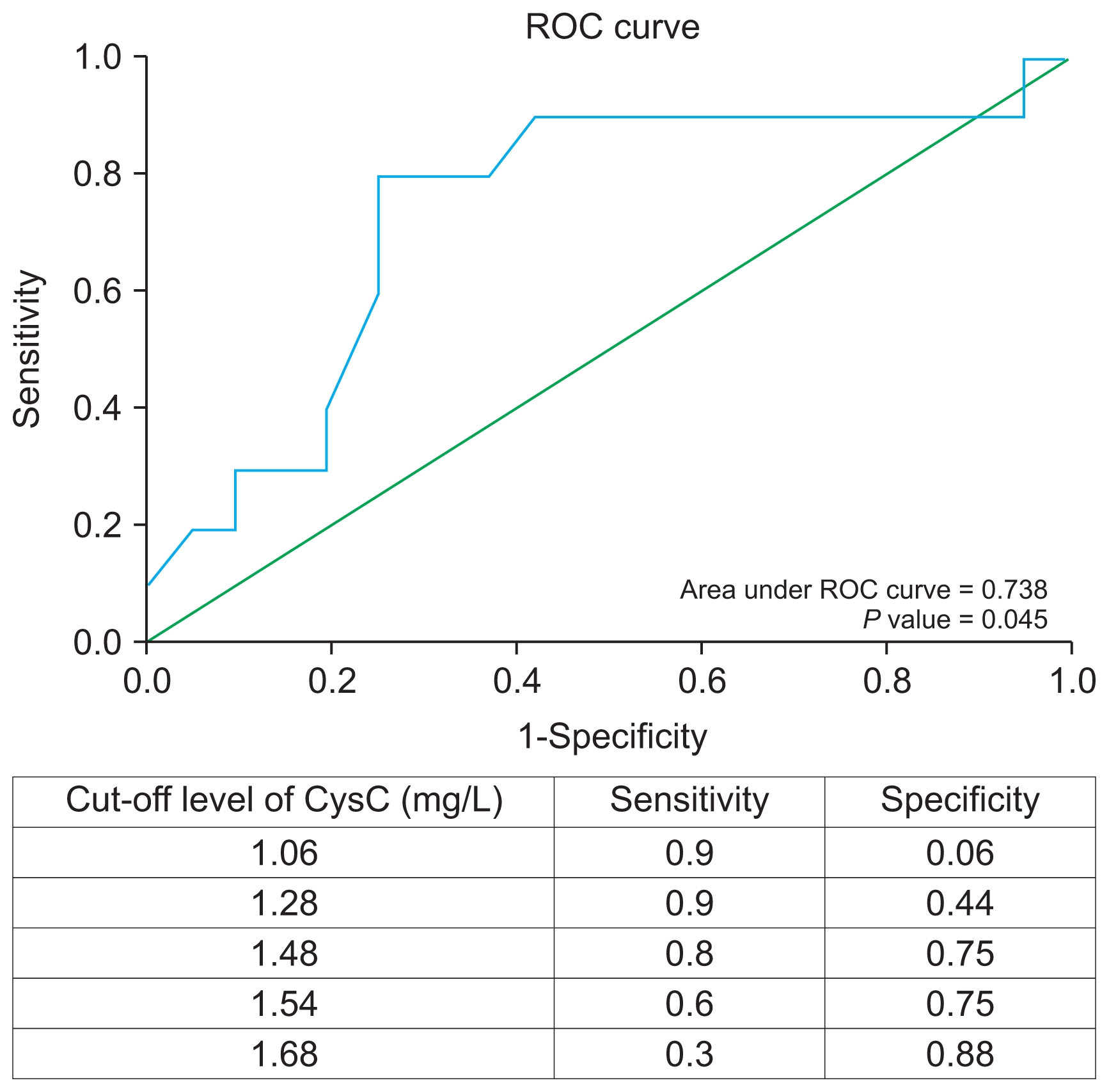
Table 1
Characteristics of pregnant women with severe preeclampsia
| Clinical variable | Value |
|---|---|
| Subject (n) | 26 |
| Maternal age (yr) | 29.9 ± 7.6 |
| Teenage pregnancy* | 3 (11.5) |
| Elderly pregnancy† | 7 (26.9) |
| Systolic blood pressure (mmHg) | 167.5 ± 12.1 (160–220) |
| Diastolic blood pressure (mmHg) | 104.9± 11.2 (90–140) |
| Gestational age at delivery (wk) | 37.1 ± 2.3 |
| Preterm delivery | 10 (38.5) |
| Low birth weight‡ | 5 (19.2) |
| Placental weight (g) | 571.5 ± 149.4 (371.1–913.0) |
| Serum creatinine (mg/dL) | 0.6 ± 0.1 (0.40–0.79) |
| Serum cystatin C (mg/L) | 1.4 ± 0.2 (0.99–1.86) |
| Hematologic profile | |
| Hemoglobin (g/dL) | 12.0 ± 1.2 |
| Platelets (×103/mm3) | 275.1 ± 92.4 |
| LDH (U/L) (normal, 125–220) | 204.3 ± 65.3 |
| PT (sec) (normal, 12–14.6) | 11.9 ± 0.8 |
| INR (normal, 0.8–1.2) | 1.0 ± 0.1 |
| aPTT (sec) (normal, 25.1–35.1) | 27.8 ± 3.0 |
| Liver function tests | |
| AST (U/L) (normal, 5–34) | 19.9 ± 7.9 |
| ALT (U/L) (normal, 0–54) | 14.0 ± 7.4 |
Data are presented as number only, mean ± standard devation (range), or number (%). ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate transaminase; INR, international normalized ratio; LDH, lactate dehydrogenase; PT, prothrombin time.
‡ Low birth weight corrected by gestational age of a fetal-growth chart developed for Thai pregnant women (Kiserud et al [30]).
Table 2
Correlation between glomerular filtration rate (GFR) estimated using different biomarkers and equations
| Equation | ||||
|---|---|---|---|---|
|
|
||||
| CKD-EPI | MDRD | Cockcroft and Gault | CysC | |
| CKD-EPI creatinine at time 0 | 0.91** | 0.75** | 0.41* | |
| MDRD at time 0 | 0.91** | 0.81** | 0.52** | |
| Cockcroft and Gault at time 0 | 0.75** | 0.81** | 0.45* | |
| CysC-GFR | 0.41* | 0.52** | 0.45* | |
Table 3
Average individual eGFR calculated using various equations
Table 4
Binary logistic regression analysis for predicting preterm delivery and low birth weight adjusted by maternal age and systolic and diastolic blood pressure
References
1. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010 age-period-cohort analysis. BMJ 347:f65642013;



2. Saleem S, McClure EM, Goudar SS, et al. A prospective study of maternal, fetal and neonatal deaths in low- and middle-income countries. Bull World Health Organ 1:605–612. 2014;

3. Souza JP, Gülmezoglu AM, Vogel J, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet 381:1747–1755. 2013;

4. Hansen AR, Barnés CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr 156:532–536. 2010;


5. Strand KM, Heimstad R, Iversen AC, et al. Mediators of the association between pre-eclampsia and cerebral palsy: population based cohort study. BMJ 347:f40892013;



6. Prakash J, Ganiger VC, Prakash S, et al. Acute kidney injury in pregnancy with special reference to pregnancy-specific disorders: a hospital based study 2014–2016. J Nephrol 31:79–85. 2018;


7. Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 10:147–155. 2015;


8. Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 48:463–493. 2008;



10. Kreepala C, Luangphiphat W, Villarroel A, Kitporntheranunt M, Wattanavaekin K, Piyajarawong T. Effect of magnesium on glomerular filtration rate and recovery of hypertension in women with severe preeclampsia. Nephron 138:35–41. 2018;


11. Kreepala C, Kitporntheranunt M, Sangwipasnapaporn W, Rungsrithananon W, Wattanavaekin K. Assessment of pre-eclampsia risk by use of serum ionized magnesium-based equation. Ren Fail 40:99–106. 2018;



12. Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 54:2056–2063. 1998;


13. Huang C, Chen S. Acute kidney injury during pregnancy and puerperium: a retrospective study in a single center. BMC Nephrol 18:1462017;



14. Smith MC, Moran P, Ward MK, Davison JM. Assessment of glomerular filtration rate during pregnancy using the MDRD formula. BJOG 115:109–112. 2008;


15. Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol 22:810–820. 2011;


16. Filler G, Bökenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A. Cystatin C as a marker of GFR—history, indications, and future research. Clin Biochem 38:1–8. 2005;


17. Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children--a meta-analysis. Clin Biochem 40:383–391. 2007;


18. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40:221–226. 2002;


19. Zappitelli M, Greenberg JH, Coca SG, et al. Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr 169:583–591. 2015;



20. Yalcin S, Ulas T, Eren MA, et al. Relationship between oxidative stress parameters and cystatin C levels in patients with severe preeclampsia. Medicina (Kaunas) 49:118–123. 2013;


21. Niraula A, Lamsal M, Baral N, et al. Cystatin-C as a marker for renal impairment in preeclampsia. J Biomark 2017:7406959. 2017

22. Abitbol CL, Seeherunvong W, Galarza MG, et al. Neonatal kidney size and function in preterm infants: what is a true estimate of glomerular filtration rate? J Pediatr 164:1026–1031. 2014;


23. Gursoy AY, Tasci Y, Celik H, et al. The prognostic value of first-trimester cystatin C levels for gestational complications. J Perinat Med 44:295–299. 2016;


24. Risch M, Purde MT, Baumann M, et al. High first-trimester maternal blood cystatin C levels despite normal serum creatinine predict pre-eclampsia in singleton pregnancies. Scand J Clin Lab Invest 77:634–643. 2017;


25. Buderer NM. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med 3:895–900. 1996;


26. Ngwenya S. Severe preeclampsia and eclampsia: incidence, complications, and perinatal outcomes at a low-resource setting, Mpilo Central Hospital, Bulawayo, Zimbabwe. Int J Womens Health 9:353–357. 2017;



28. Howson CP, Kinney MV, McDougall L, Lawn JE. Born Too Soon Preterm Birth Action Group. Born too soon: preterm birth matters. Reprod Health 10:Suppl 1. S12013;



29. Lee AC, Kozuki N, Cousens S, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21st standard: analysis of CHERG datasets. BMJ 358:j36772017;



30. Kiserud T, Benachi A, Hecher K, et al. The World Health Organization fetal growth charts: concept, findings, interpretation, and application. Am J Obstet Gynecol 218:S619–S629. 2018;


31. Al-Turki HA, Abu-Heija AT, Al-Sibai MH. The outcome of pregnancy in elderly primigravidas. Saudi Med J 24:1230–1233. 2003;

32. Wall-Wieler E, Roos LL, Nickel NC. Adolescent pregnancy outcomes among sisters and mothers: a population-based retrospective cohort study using linkable administrative data. Public Health Rep 133:100–108. 2018;


33. Inker LA, Schmid CH, Tighiouart H, et al. CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367:20–29. 2012;



34. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. 2009;



35. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254. 2006;


36. National Kidney Foundation, Inc. GFR Calculator Available at: https://www.kidney.org/professionals/kdoqi/gfr_calculator.
37. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. 1976;


38. Mundt LA, Shanahan K. Chemical analysis of urine. In: Mundt LA, Shanahan K, Graff’s Textbook of Routine Urinalysis and Body Fluids. 2nd edition. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; p. 35–54. 2010.
39. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis 20:229–239. 2013;



40. National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol 183:S1–S22. 2000;

41. Padma Y, Aparna VB, Kalpana B, Ritika V, Sudhakar PR. Renal markers in normal and hypertensive disorders of pregnancy in Indian women: a pilot study. Int J Reprod Contracept Obstet Gynecol 2:514–520. 2013;



42. Strevens H, Wide-Swensson D, Grubb A. Serum cystatin C is a better marker for preeclampsia than serum creatinine or serum urate. Scand J Clin Lab Invest 61:575–580. 2001;


43. Franceschini N, Qiu C, Barrow DA, Williams MA. Cystatin C and preeclampsia: a case control study. Ren Fail 30:89–95. 2008;


44. Babay Z, Al-Wakeel J, Addar M, et al. Serum cystatin C in pregnant women: reference values, reliable and superior diagnostic accuracy. Clin Exp Obstet Gynecol 32:175–179. 2005;

45. Novakov Mikic A, Cabarkapa V, Nikolic A, et al. Cystatin C in pre-eclampsia. J Matern Fetal Neonatal Med 25:961–965. 2012;


46. Bjornstad P, Cherney DZI. Kidney function can predict pregnancy outcomes. Clin J Am Soc Nephrol 12:1029–1031. 2017;



47. Piccoli GB, Attini R, Vasario E, et al. Pregnancy and chronic kidney disease: a challenge in all CKD stages. Clin J Am Soc Nephrol 5:844–855. 2010;



48. Sturgiss SN, Wilkinson R, Davison JM. Renal reserve during human pregnancy. Am J Physiol 271(1 Pt 2):F16–F20. 1996;


49. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol 6:2587–2598. 2011;



50. Piccoli GB, Cabiddu G, Attini R, et al. Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol 26:2011–2022. 2015;



51. Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65:1416–1421. 2004;


52. Chu SC, Wang CP, Chang YH, et al. Increased cystatin C serum concentrations in patients with hepatic diseases of various severities. Clin Chim Acta 341:133–138. 2004;


53. Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int 63:1944–1947. 2003;


54. Wiesli P, Schwegler B, Spinas GA, Schmid C. Serum cystatin C is sensitive to small changes in thyroid function. Clin Chim Acta 338:87–90. 2003;


55. Tan GD, Lewis AV, James TJ, et al. Clinical usefulness of cystatin C for the estimation of glomerular filtration rate in type 1 diabetes: reproducibility and accuracy compared with standard measures and iohexol clearance. Diabetes Care 25:2004–2009. 2002;


56. Xia LH, Bing XG, An XT. Serum cystatin C assay for the detection of early renal impairment in diabetic patients. J Clin Lab Anal 18:31–35. 2004;



- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















