| Kidney Res Clin Pract > Volume 35(2); 2016 > Article |
|
Abstract
Background
Renal infarction (RI) is an uncommon disease that is difficult to diagnose. As little is known about clinical characteristics of this disease, we investigated its underlying risk factors and outcomes.
Methods
We performed a retrospective single-center study of 89 patients newly diagnosed with acute RI between January 2002 and March 2015 using imaging modalities. Clinical features, possible etiologies, and long-term renal outcome data were reviewed.
Results
The patients' mean age was 63.5┬Ā┬▒┬Ā15.42 years; 23.6% had diabetes and 56.2% had hypertension. Unilateral and bilateral involvements were shown in 80.9% and 19.1% of patients, respectively; proteinuria and hematuria were reported in 40.4% and 41.6%, respectively. Cardiovascular disease was the most common underlying disease, followed by renal vascular injury and hypercoagulability disorder. Fourteen patients had no specific underlying disease. At the time of diagnosis, acute kidney injury (AKI) was found in 34.8% of patients. Univariate analysis revealed diabetes mellitus (DM), leukocytosis, and high C-reactive protein (CRP) as significant risk factors for the development of AKI. On multivariate analysis, DM and high CRP levels were independent predictors for AKI. During follow-up, chronic kidney disease developed in 27.4% of patients. Univariate and multivariate Cox regression analyses showed old age to be an independent risk factor for this disease, whereas AKI history was a negative risk factor.
Conclusion
DM patients or those with high CRP levels should be observed for renal function deterioration. Clinicians should also monitor for RI in elderly patients.
Keywords
Acute kidney injury, Chronic kidney disease, Infarction, Renal arteryRenal infarction (RI) is an infrequent condition that is related to atheroembolic or thromboembolic disease [1]. The first case of renal embolic disease was reported in 1856 [2], and several more cases and clinical analyses have been published since. Most reported cases have underlying causes, such as hypercoagulability, cardiac problems, trauma history, or previous cerebral infarction [2], [3], [4]. Various comorbidities, such as infection, malignancy, cocaine abuse, and autoimmune disease, may also contribute to RI [5], [6], [7], [8], [9]. Abdominal or flank pain is a common clinical manifestation, although some patients do not experience any subjective symptoms. Therefore, RI is difficult to detect unless a clinician specifically checks for its presence┬Āand is thus likely to be underdiagnosed [1], [3], [4], [10], [11], [12]. Postmortem examinations in 1 study revealed that the incidence of RI was 1.4% (205 of 14,411) with only 2 of these cases having been clinically diagnosed antemortem [13]. Another study reported an RI incidence of 0.007% during a 36-month observation period [14].
With the advent of innovative imaging technologies, incidental RI cases are detected more frequently [4]. Improved accessibility to contrast-enhanced computed tomography can also reveal more RI cases. Nowadays, RI can be detected in patients who visit hospitals with nonspecific symptoms and no demonstrable underlying causes.
A partial RI animal model induced by renal artery ligation revealed increased plasma renin activity and development of hypertension [15], but there are no firm data regarding long-term follow-up results in patients after RI. Reduced kidney blood flow and mass reduction can cause deterioration of renal function, although hyperperfusion of the viable renal glomeruli can compensate┬Āfor this [1]. In this context, RI may be thought of as a completely reversible disease. However, there are also patients who progress to a state where they require dialysis [8].
The purpose of this study was 2-fold. First, we aimed to reveal the clinical etiology and characteristics of RI. Second, we investigated the acute and long-term changes in renal function due to RI┬Āand determined the factors associated with renal functional deterioration.
This was a retrospective, single-center study of 89 patients newly diagnosed with acute RI between January 2002 and March 2015. Diagnosis was based on a radiologist's report of imaging modalities, such as computed tomography, sonography, and angiography. Patients who visited the emergency department or underwent imaging studies during hospitalization were included. The creatinine level measured 7ŌĆō365 days before the study was considered as the baseline value [16]. If there were no preexisting creatinine data, baseline creatinine was regarded as the healthiest creatinine level measured. Patients younger than 18 years┬Āwere excluded. Clinical manifestations and possible etiologies were extracted from initial emergency room medical records or admission notes. We used the Risk, Injury, Failure, Loss, and End-stage kidney disease criteria to define acute kidney injury (AKI) as measured by a change in serum creatinine levels within 1ŌĆō7 days from the initial renal insult [17]. Development of chronic kidney disease (CKD) was defined as a creatinine-based estimated glomerular filtration rate (eGFR) <┬Ā60┬ĀmL/min/1.73┬Ām2 over 3 months┬Āusing the Modification of Diet in Renal Disease equation [18]. For long-term outcome analysis, we excluded patients whose eGFRs were <┬Ā60┬ĀmL/min/1.73┬Ām2 at baseline. Patients who have more than 1 underlying medical condition were assessed by using Charlson comorbidity index (CCI) score [19]. This study was approved by the Korea University Anam Hospital Institutional Review Board (2015-04-0345).
The IBM SPSS statistics software, version 20 (IBM analytics, Chicago, IL, USA) was used for statistical analyses. Comparison between 2 groups was performed using the t test for numerical data and the chi-square test or Fisher exact test for categorical data. Univariate logistic tests were conducted for AKI and RI. Results that followed normal distribution were presented as mean values. In contrast, if the results did not follow normal distribution, the median value was used. We used multivariate Cox regression tests to identify predictors of long-term decreased renal function because of RI diagnosis. All demographic factors, comorbidities, laboratory data, treatment options, and first manifestations of symptoms were included in the list of possible risk factors for decreasing renal function.
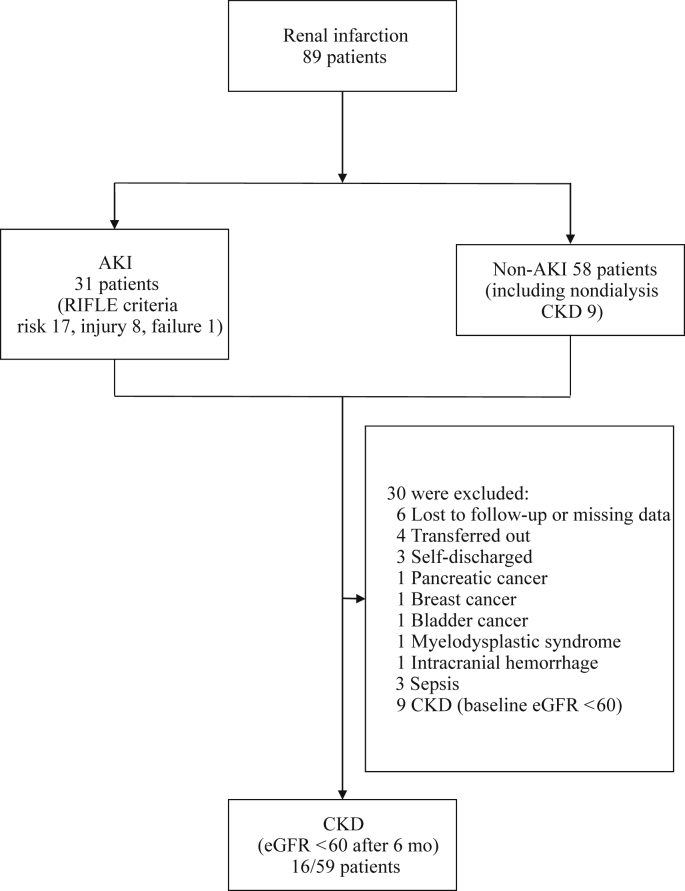
A total of 89 acute RI patients were identified during the 13┬Āyears. The mean follow-up period was 33.45┬Ā┬▒┬Ā29.04 months with a maximum of 119 months. Six patients had no medical record because of discharge or follow-up loss (Fig.┬Ā1). Four patients transferred to another hospital before 3 months had died, whereas 3 patients were excluded from the analysis of long-term renal function because of self-discharge. Five patients died of pancreatic cancer, breast cancer, bladder cancer, myelodysplastic syndrome, and intracranial hemorrhage, respectively, whereas 3 patients died of sepsis. Nine patients exhibited reduced renal function at the first follow-up time point with an eGFR below 60┬ĀmL/min/1.73┬Ām2. Hence, 59 patients were ultimately included for analysis of the long-term change in renal function at 1 year or more after RI diagnosis.
AKI was found in 34.8% of patients (31 cases) at the time of diagnosis (Table┬Ā1). According to the Risk, Injury, Failure, Loss, and End-stage kidney disease criteria, 17 (19.7%) patients were at risk, 8 (9.3%) patients had injury, and 1 (1.1%) patient┬Āhad kidney failure. AKI patients were older than those without AKI, although the difference was not significant. More AKI patients had diabetes mellitus (DM) (P┬Ā=┬Ā0.010), were current or former smokers (P┬Ā=┬Ā0.010), showed more hematuria (P┬Ā=┬Ā0.020), and had lower baseline eGFR (P┬Ā=┬Ā0.050), severe leukocytosis (P┬Ā=┬Ā0.010), and higher C-reactive protein (CRP) levels (P┬Ā=┬Ā0.010). Cardiovascular disease was the most common risk factor (38.2%), followed by renal vascular injury and hypercoagulability disorder (14.6% and 3.4%, respectively). Hypercoagulability conditions included 1 antiphospholipid syndrome patient and 2 lowŌĆōprotein C patients. Cardiovascular diseases included atrial fibrillation (22; 24.7%), valvular heart disease (4; 4.4%), endocarditis (4; 4.4%), stable angina (3; 3.3%), and pulmonary thromboembolism (1; 1.1%). We also observed additional comorbidities that may be risk factors for thromboembolism associated with RI (provided in the footnote of Table┬Ā1). Moreover, 10 (11.2%) of the cases were idiopathic. The average CCI that represents the comorbid condition was 2. Abdominal pain was the most frequently experienced symptom (41.6%), whereas 25.8% of patients were asymptomatic. We observed synchronous infarctions of other organs in 10 (11.2%) patients; the most common site was the spleen (5 splenic infarctions and 1 segmental splenic infarction with descending colon infarction) followed by combined bowel ischemia (2 diffuse ischemic colitis and 2 small bowel ischemia cases). Bilateral renal artery involvement was observed in 30 (33.7%) patients. Regarding treatment, antiplatelet therapy, anticoagulation, and thrombolysis (for uncontrolled abdominal pain) were administered to 47 (52.8%), 54 (60.7%), and 2 (2.2%) patients, respectively. Incidences of DM, malignancy, and AKI; CCI score; and CRP levels were significantly different in the patients who were excluded from CKD analysis (Table┬Ā2). Initial CKD patients were older, had lower baseline eGFR, and had a higher smoking rate (Table┬Ā3).
On univariate analysis, DM (P┬Ā=┬Ā0.005), high CRP (P┬Ā=┬Ā0.001), and leukocytosis (P┬Ā=┬Ā0.007) showed a significant relationship with AKI (Table┬Ā4). On multivariate analysis, DM (hazard ratio [HR], 4.424; P┬Ā=┬Ā0.023) and high CRP level (HR, 1.016; P┬Ā=┬Ā0.008) were independent risk factors for AKI (Table┬Ā4). CKD developed in 16 cases (27.1%) during the follow-up period. None of the 59 patients who were initially non-CKD required renal replacement therapy. On univariate Cox regression analysis, old age (HR, 1.085; P┬Ā=┬Ā0.004) was an independent predictor for eGFR decline (Table┬Ā5). We divided RI patients into 2 groups: one including those older than 60 years with an annual eGFR decrease of 1.416┬Ā┬▒┬Ā5.35┬ĀmL/min/1.73┬Ām2 and the other of patients younger than 60 years with eGFR decreasing at a rate of 1.16┬Ā┬▒┬Ā7.92┬ĀmL/min/1.73┬Ām2. AKI history showed an independent negative relationship with CKD development on univariate Cox regression analysis (P┬Ā=┬Ā0.021), although the odds ratio was 0.161, indicating that RI patients who experienced AKI showed less severe long-term renal outcomes. Multivariate analysis consistently showed that advanced age was a significant independent risk factor for CKD progression (P┬Ā=┬Ā0.017). On multivariate analysis, AKI history also showed similar significance (P┬Ā=┬Ā0.014; Table 5).
One-third of RI patients in our study showed AKI on initial presentation, and a quarter subsequently developed CKD. Most patients had specific clinical manifestation of symptoms on initial presentation. No deceased or end-stage renal disease patients required renal replacement therapy because of RI. DM and high CRP levels were risk factors for AKI; moreover, although old age was a risk factor for CKD, a history of AKI showed a protective effect.
A high CRP level was an independent risk factor for AKI after RI. CRP is indicative of a systemic inflammatory burden and local tissue injury. CRP is also used as an inflammatory marker in various other diseases [20], [21], [22]. It can cause secondary systematic inflammation and tissue damage owing to its induction of cytokines [20]. The high level of CRP may reflect the proportion of renal damage caused by RI; as CRP levels can directly reflect the level of destruction to the renal parenchyma, it can be used as a marker of reduced kidney function.
Patients with DM may experience more severe renal function degeneration on acute presentation. DM remains an independent predictor for AKI in RI patients, even after adjusting for the CRP level. Underlying diabetes is one of the risk factors of poor outcome in many diseases [23], [24], [25], [26]. Insulin resistance can cause severe metabolic derangement and poor outcome, whereas metabolic dysregulation in DM patients, linked with sustained systemic inflammation [27], can cause macrovascular and microvascular damage. Such vulnerable vasculature can lead to ischemic insult┬Āand subsequently to severe functional deterioration.
Old age was an independent predictor for CKD. Decline in renal function begins after the fourth decade, and the slope of creatinine clearance approaches ŌĆō1.24┬Ā┬▒┬Ā0.3 in normal healthy subjects older than 60┬Āyears [28]. Individual glomeruli deteriorate functionally and structurally, and such loss of renal mass with aging results in a decreased eGFR. A preexisting aging-related decrease in renal function combined with a new insult caused by RI inevitably leads to a poor┬Āand irreversible outcome.
Reversible AKI is a risk factor for de novo CKD, mortality, and it is connected with the severity [29], [30], [31]. In contrast, we found that AKI with RI was negatively related to CKD progression. The long-term analysis was only conducted with patients who survived. As we discussed in Table┬Ā2, excluded patients experienced more AKI, but they are not tracked and dead. They showed higher incidence of DM, malignancy, and upper CCI score. This specificity might contribute to fetal outcome. On the other hand, patients with a history of AKI may undergo more intensive monitoring┬Āand thus be at lower risk of developing CKD. We found small differences on detailed subanalysis that were not detected during overall analysis. Patients who experienced AKI without CKD development have more cardiogenic risk factors (59% vs. 32.8%, respectively; P┬Ā=┬Ā0.040) and a higher DM incidence (45.5% vs. 16.7%, respectively; P┬Ā=┬Ā0.010) than those who developed no CKD. A greater proportion had also undergone combined antiplatelet and anticoagulation therapy, although the difference was not statistically significant (46.3% vs. 22.7%, respectively; P┬Ā=┬Ā0.070). Patients at risk who develop AKI should consider dual-agent therapy to prevent CKD.
According to Oh et┬Āal [32] and Bae et┬Āal [33], cardiogenic risk factors, especially atrial fibrillation, are a main cause of RI. We found cardiogenic etiologies to be the most common single cause of RI (38.2% of cases). We investigated an expanded list of factors that play a role in systemic inflammation as well as vascular and/or coagulation disorders that may lead to infarction. Various conditions were incorporated into the CCI; however, CCI did not show a significant relationship with AKI or CKD development.
Infarctions in other organs that were observed in 10 of our patients at the time of diagnosis, and in 7 additional patients thereafter, were associated with more than 1 risk factor in our patients (including cancer plus each of atrial fibrillation, endocarditis, hypertension, DM, malaria, or cerebral infarction history, as well as DM plus a postoperative immobilization state). Intensive anticoagulation therapy and careful probing for underlying disease may be required to prevent concomitant adverse thromboembolic events as suggested by Yun [10]; however, this could result in more adverse outcomes in certain patients such as those with cancer. Infarctions in other organs are not associated with renal function change.
This study has several limitations. We did not measure the infarction size. We attempted to measure the infarction size but faced technical limitations. In our institute, volumetry is only available when using a special computed tomography protocol, which is different from the protocols we used. Furthermore, the study was a retrospective, observational, and single-center study, which would have led to bias in the data. For more accurate measurements of inflammatory burdens, other cytokines and biomarkers ought to be evaluated in addition to CRP. More than one-third of our patients were excluded from CKD analysis for various reasons as mentioned previously. Finally, underlying conditions were not fully evaluated in some patients, especially those with idiopathic RI; hence, more extensive investigation strategies are required in such cases. To definitively determine if RI is a risk factor for CKD, a comparative study by using propensity-matched healthy subjects is advisable. Moreover, a multicenter large-scale study is necessary to identify hidden risk factors for idiopathic RI.
In conclusion, we found that 34.8% of patients diagnosed with RI also experienced AKI, and 27.1% experienced CKD during the follow-up period. Most patients had clinical symptoms and underlying risk factors. Risk factors for AKI were DM and increased CRP levels, which may be indicative of severe damage. Old age was a risk factor for CKD, whereas AKI was not a risk factor for CKD. Medical therapy was sufficient in most cases. Some patients with multiple comorbidities also exhibited infarctions in other organs. Finally, AKI history is inversely proportional to the deterioration of eGFR.
References
1. Longo D.L., Fauci A.S., Kasper D.L., Hauser S.L., Jameson J.L., Loscalzo J.. Harrison's Principles of Internal Medicine. 18th edition. 2012. McGraw-Hill Companies; New York (NY).
2. Lessman R.K., Johnson S.F., Coburn J.W., Kaufman J.J.. Renal artery embolism: clinical features and long-term follow-up of 17 cases. Ann Intern Med 89:1978;477ŌĆō482.


3. Bourgault M., Grimbert P., Verret C., Pourrat J., Herody M., Halimi J.M., Karras A., Amoura Z., Jourde-Chiche N., Izzedine H., Fran├¦ois H., Boffa J.J., Hummel A., Bernadet-Monrozies P., Fouque D., Canou├»-Poitrine F., Lang P., Daugas E., Audard V.. Acute renal infarction: a case series. Clin J Am Soc Nephrol 8:2013;392ŌĆō398.


4. Antopolsky M., Simanovsky N., Stalnikowicz R., Salameh S., Hiller N.. Renal infarction in the ED: 10-year experience and review of the literature. Am J Emerg Med 30:2012;1055ŌĆō1060.


6. Th├®riault J., Agharazzi M., Dumont M., Pichette V., Ouimet D., Leblanc M.. Atheroembolic renal failure requiring dialysis: potential for renal recovery? A review of 43 cases. Nephron Clin Pract 94:2002;c11ŌĆōc18.

7. Mochizuki Y., Zhang M., Golestaneh L., Thananart S., Coco M.. Acute aortic thrombosis and renal infarction in acute cocaine intoxication: a case report and review of literature. Clin Nephrol 60:2003;130ŌĆō133.


8. Kim J.S., Lee S.Y., Kim J.H., Kwon E.H., Song S.H., Lee D.W., Lee S.B., Kwak I.S.. Acute renal infarction: clinical features in 23 cases. Korean J Med 70:2006;543ŌĆō550.
9. Madhrira M.M., Mohan S., Markowitz G.S., Pogue V.A., Cheng J.T.. Acute bilateral renal infarction secondary to cocaine-induced vasospasm. Kidney Int 76:2009;576ŌĆō580.


10. Yun W.S.. Long-term follow-up results of acute renal embolism after anticoagulation therapy. Ann Vasc Surg 29:2015;491ŌĆō495.


11. Huang C.C., Chen W.L., Chen J.H., Wu Y.L., Shiao C.J.. Clinical characteristics of renal infarction in an Asian population. Ann Acad Med Singapore 37:2008;416ŌĆō420.


12. Kim S.C., Cha J.J., Oh S.W., Kwon O.S., Kang Y.S., Kim H.K., Cha D.R.. A case of renal infarct developed in acute pancreatitis. Korean J Nephrol 28:2009;350ŌĆō354.
13. Hoxie H.J., Coggin C.B.. Renal infarction: statistical study of two hundred and five cases and detailed report of an unusual case. Arch Intern Med 65:1940;587ŌĆō594.
14. Korzets Z., Plotkin E., Bernheim J., Zissin R.. The clinical spectrum of acute renal infarction. Isr Med Assoc J 4:2002;781ŌĆō784.

15. Norman R.A. Jr., Galloway P.G., Dzielak D.J., Huang M.. Mechanisms of partial renal infarct hypertension. J Hypertens 6:1988;397ŌĆō403.


16. Siew E.D., Ikizler T.A., Matheny M.E., Shi Y., Schildcrout J.S., Danciu I., Dwyer J.P., Srichai M., Hung A.M., Smith J.P., Peterson J.F.. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 7:2012;712ŌĆō719.



17. Kdigo A.. Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:2012;1ŌĆō138.
18. Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D.. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:1999;461ŌĆō470.


19. Kastner C., Armitage J., Kimble A., Rawal J., Carter P.G., Venn S.. The Charlson comorbidity score: a superior comorbidity assessment tool for the prostate cancer multidisciplinary meeting. Prostate Cancer Prostatic Dis 9:2006;270ŌĆō274.


20. Karadag F., Kirdar S., Karul A.B., Ceylan E.. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med 19:2008;104ŌĆō108.


21. Stuveling E.M., Hillege H.L., Bakker S.J., Gans R.O., De Jong P.E., De Zeeuw D.. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int 63:2003;654ŌĆō661.


22. Podzimek S., Mysak J., Janatova T., Duskova J.. C-reactive protein in peripheral blood of patients with chronic and aggressive periodontitis, gingivitis, and gingival recessions. Mediators Inflamm 2015:2015;564858



23. O'Carroll G., Kearney P.P.. Diabetes mellitus in percutaneous coronary intervention: greater awareness is needed to predict and prevent poor outcomes. EuroIntervention 10:2014;13ŌĆō15.


24. Aikens J.E.. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care 35:2012;2472ŌĆō2478.




25. Hadden D.R., Cull C.A., Croft D.J., Holman R.R.. Poor pregnancy outcome for women with type 2 diabetes. Diabet Med 20:2003;506ŌĆō507.


26. Jia Q., Zhao X., Wang C., Wang Y., Yan Y., Li H., Zhong L., Liu L., Zheng H., Zhou Y., Wang Y.. Diabetes and poor outcomes within 6 months after acute ischemic stroke: the China National Stroke Registry. Stroke 42:2011;2758ŌĆō2762.


27. Wellen K.E., Hotamisligil G.S.. Inflammation, stress, and diabetes. J Clin Invest 115:2005;1111ŌĆō1119.



28. Glassock R.J., Winearls C.. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc 120:2009;419ŌĆō428.


29. Chawla L.S., Amdur R.L., Amodeo S., Kimmel P.L., Palant C.E.. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79:2011;1361ŌĆō1369.



30. Bucaloiu I.D., Kirchner H.L., Norfolk E.R., Hartle J.E. 2nd, Perkins R.M.. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81:2012;477ŌĆō485.


31. Coca S.G., Singanamala S., Parikh C.R.. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81:2012;442ŌĆō448.


Figure┬Ā1
Flowchart of patient outcome.
AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; RIFLE, Risk, Injury, Failure, Loss, and End-stage kidney disease.
Table┬Ā1
Baseline characteristics of renal infarction patients in the AKI versus non-AKI groups
| Total (n┬Ā=┬Ā89) | AKI (n┬Ā=┬Ā31) | Non-AKI (n┬Ā=┬Ā58) | P | |
|---|---|---|---|---|
| Age, median (minimumŌĆōmaximum) | 60.9 (21.0ŌĆō87.0) | 60.0 (21.0ŌĆō84.0) | 68.0 (25.0ŌĆō87.0) | 0.070 |
| Male | 53.0 (59.6) | 21.0 (67.7) | 32.0 (55.2) | 0.370 |
| DM | 21.0 (23.6) | 13.0 (38.5) | 8.0 (18.3) | 0.010ŌłŚ |
| Hypertension | 50.0 (56.0) | 15.0 (48.4) | 23.0 (39.7) | 0.530 |
| Smoking | 37.0 (41.6) | 16.0 (51.6) | 21.0 (36.2) | 0.010ŌłŚ |
| BMI (kg/m2) | 23.5 (13.0ŌĆō32.0) | 24.0 (13.0ŌĆō32.0) | 24.0 (15.0ŌĆō31.0) | 0.840 |
| Proteinuria | 36.0 (40.4) | 14.0 (46.1) | 22.0 (37.9) | 0.490 |
| Hematuria | 37.0 (41.6) | 18.0 (58.1) | 19.0 (32.6) | 0.020ŌłŚ |
| Baseline eGFR, median (minimumŌĆōmaximum) | 80.7 (26.2ŌĆō177.8) | 84.4 (35.0ŌĆō177.8) | 75.8 (26.2ŌĆō119.0) | 0.010ŌłŚ |
| Risk factors | ||||
| ┬ĀCardiovascularŌĆĀ | 34.0 (38.2) | 10.0 (32.3) | 24.0 (41.3) | 0.740 |
| ┬ĀCoagulation | 3.0 (3.4) | 1.0 (3.2) | 2.0 (3.4) | 0.950 |
| ┬ĀRenal vascular injury | 13.0 (14.6) | 4.0 (12.9) | 9.0 (15.5) | 0.730 |
| ┬ĀOther conditionsŌĆĪ | 45.0 (50.6) | 20.0 (64.5) | 25.0 (43.1) | 0.250 |
| ┬ĀIdiopathic | 10.0 (11.2) | 2.0 (6.7) | 8.0 (13.6) | 0.480 |
| ┬ĀCCI | 2.0 (0.0ŌĆō10.0) | 2.0 (0ŌĆō10.0) | 1.0 (0ŌĆō9) | 0.320 |
| ┬ĀCombined other organ infarction | 10.0 (11.2) | 5.0 (16.1) | 5.0 (8.6) | 0.310 |
| ┬ĀBilateral involvement | 30.0 (33.7) | 6.0 (20.0) | 24.0 (80.0) | 0.540 |
| Treatment option | ||||
| ┬ĀAntiplatelet┬Āalone | 11.0 (12.3) | 6.0 (19.3) | 5.0 (8.6) | 0.180 |
| ┬ĀAnticoagulation alone | 18.0 (20.2) | 4.0 (12.9) | 14.0 (24.1) | 0.270 |
| ┬ĀASA┬Ā+┬ĀWFR | 36.0 (40.4) | 10.0 (32.3) | 26.0 (44.8) | 0.260 |
| ┬ĀThrombolysis | 2.0 (2.2) | 1.0 (3.2) | 1.0 (1.7) | 0.950 |
| Symptoms | ||||
| ┬ĀAbdominal pain | 37.0 (41.6) | 10.0 (32.3) | 27.0 (46.5) | ŌĆō |
| ┬ĀFlank pain | 13.0 (14.6) | 4.0 (12.9) | 9.0 (15.5) | ŌĆō |
| ┬ĀFever | 9.0 (10.1) | 2.0 (6.4) | 4.0 (6.8) | ŌĆō |
| ┬ĀNausea | 1.0 (1.1) | 0 | 1.0 (1.7) | ŌĆō |
| ┬ĀDyspnea | 3.0 (3.4) | 1.0 (3.2) | 2.0 (3.4) | ŌĆō |
| ┬ĀNo symptoms | 23.0 (25.8) | 5.0 (16.1) | 18.0 (31.1) | ŌĆō |
| WBC | 10,239┬Ā┬▒┬Ā6,376 | 12,963┬Ā┬▒┬Ā8,962 | 8,675┬Ā┬▒┬Ā3,485 | 0.010ŌłŚ |
| Hb, median (minimumŌĆōmaximum) | 13.1 (7.1ŌĆō21.7) | 14.0 (8.0ŌĆō21.7) | 13.0 (7.1ŌĆō18.0) | 0.930 |
| Plt(k), median (minimumŌĆōmaximum) | 212.0 (16.0ŌĆō484.0) | 207.0 (16.0ŌĆō484.0) | 190.0 (16.0ŌĆō363.0) | 0.460 |
| CRP | 61.3┬Ā┬▒┬Ā71.9 | 108.9┬Ā┬▒┬Ā79.9 | 35.2┬Ā┬▒┬Ā51.6 | 0.010ŌłŚ |
| LDH | 1,104┬Ā┬▒┬Ā826 | 1,211┬Ā┬▒┬Ā896 | 1,035┬Ā┬▒┬Ā784 | 0.430 |
| HDL, median (minimumŌĆōmaximum) | 41.0 (10.0ŌĆō83.0) | 41.5 (10.0ŌĆō83.0) | 41.0 (12.0ŌĆō63.0) | 0.770 |
| LDL, median (minimumŌĆōmaximum) | 90.0 (29.0ŌĆō296.0) | 91.0 (36.0ŌĆō296.0) | 87.5 (29.0ŌĆō162.0) | 0.960 |
| Uric acid, median (minimumŌĆōmaximum) | 4.8 (2.2ŌĆō7.5) | 4.6 (2.2ŌĆō7.5) | 5.2 (2.8ŌĆō7.4) | 0.430 |
| Total calcium, median (minimumŌĆōmaximum) | 8.8 (7.4ŌĆō10.5) | 8.8 (7.4ŌĆō10.5) | 8.8 (7.7ŌĆō10.1) | 0.820 |
AKI, acute kidney injury; ASA, aspirin; BMI, body mass index; CCI, Charlson comorbidity index; CRP, C-reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HDL, high-density lipoprotein; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; Plt, platelet; WBC, white blood cell; WFR, warfarin.
ŌĆĪ Other conditions include cerebral infarction, cerebral hemorrhage, cancer, rheumatoid arthritis, alcoholic hepatitis, Grave's disease, malaria, gastric ulcer perforation, postoperative state, adult Still's disease, glomerulonephritis, polycystic kidney disease, long QT syndrome, nephrotic syndrome, renal abscess, systemic lupus erythematosus, and schizophrenia.
Table┬Ā2
Clinical characteristics of excluded patients
Table┬Ā3
Clinical characteristics of patients with underlying CKD whose eGFRs were <┬Ā60┬ĀmL/min/1.73 m2
Table┬Ā4
Univariate and multivariate logistic regression analyses of risk factors associated with AKI in renal infarction patients
| Variable | Odds ratio | 95% CI | P (uni) | P (multi) |
|---|---|---|---|---|
| Age | 1.027 | 0.990ŌĆō1.065 | 0.070 | 0.156 |
| Sex | 0.632 | 0.177ŌĆō2.255 | 0.951 | ŌĆō |
| DM | 3.305 | 1.054ŌĆō10.366 | 0.005ŌłŚ | 0.040ŌłŚ |
| HTN | 1.386 | 0.574ŌĆō3.345 | 0.468 | ŌĆō |
| Smoking | 7.879 | 0.776ŌĆō4.553 | 0.164 | ŌĆō |
| CRP | 1.011 | 1.003ŌĆō1.018 | 0.001ŌłŚ | 0.004ŌłŚ |
| WBC | 1.000 | 1.000ŌĆō1.000 | 0.015ŌłŚ | 0.160 |
Table┬Ā5
Univariate and multivariate Cox regression analyses of risk factors associated with eGFR decreasing to <┬Ā60┬ĀmL/min/1.73┬Ām2 after 1 year in renal infarction patients
| Variable | Odds ratio | 95% CI | P (uni) | P (multi) |
|---|---|---|---|---|
| Age | 1.085 | 1.026ŌĆō1.148 | 0.004ŌłŚ | 0.017ŌłŚ |
| Sex | 0.958 | 0.318ŌĆō2.884 | 0.939 | ŌĆō |
| DM | 0.645 | 0.208ŌĆō1.997 | 0.447 | ŌĆō |
| HTN | 0.407 | 0.124ŌĆō1.337 | 0.138 | 0.061 |
| Comorbidities | 1.872 | 0.909ŌĆō3.856 | 0.076 | 0.063 |
| AKI history | 0.161 | 0.034ŌĆō0.763 | 0.021ŌłŚ | 0.014ŌłŚ |



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















